Long-term clinical efficacy and safety of thalidomide in patients with transfusion-dependent β-thalassemia: results from Thal-Thalido study
- PMID: 37604857
- PMCID: PMC10442319
- DOI: 10.1038/s41598-023-40849-4
Long-term clinical efficacy and safety of thalidomide in patients with transfusion-dependent β-thalassemia: results from Thal-Thalido study
Abstract
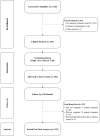
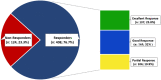
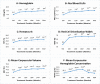
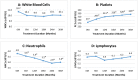
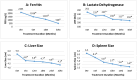
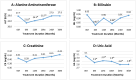
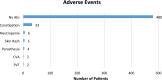
Regular blood transfusion is the mainstay of treatment in transfusion-dependent β-thalassemia (TDT); however, transfusions culminate in an array of serious complications. Therefore, a single-arm, non-randomized clinical trial was conducted in hydroxyurea refractory TDT patients to explore the long-term safety and efficacy of thalidomide. The primary outcomes for efficacy were rise in hemoglobin (Hb) level and changes in transfusion frequency. Whereas, several clinical and laboratory parameters were assessed for safety of thalidomide. Secondary outcomes included changes in serum ferritin, serum lactate dehydrogenase (LDH), serum uric acid, red blood cell indices, and size of liver and spleen. A total of 532 patients were followed for a period of 30 months. Significant increase in mean Hb level was identified at 6 months (1.4 g/dL, p ≤ 0.001) and 30 months (2 g/dL, p ≤ 0.001) in comparison with baseline. A total of 408 (76.7%) patients responded to thalidomide therapy (excellent responders 25.8%, good responders 31%, and partial responders 19.9%) and attained transfusion independence within 6 months of therapy. A significant decline in mean ferritin, LDH level, liver size, and spleen size was observed. No unfavorable effects were observed on kidney and liver functions. Mild adverse events were reported in 48 (9%) patients and serious adverse events, including cerebral vascular accident and portal vein thrombosis were reported in two patients each. This study concludes that thalidomide is an effective and well-tolerated drug that can improve Hb levels and reduce transfusion burden in hydroxyurea refractory TDT patients.Trial registration: This trial is registered at http://www.clinicaltrial.gov as # NCT03651102.
© 2023. Springer Nature Limited.
Conflict of interest statement
The authors declare no competing interests.
Figures
Similar articles
-
Comparison of efficacy and safety of thalidomide vs hydroxyurea in patients with Hb E-β thalassemia - a pilot study from a tertiary care Centre of India.Blood Cells Mol Dis. 2021 May;88:102544. doi: 10.1016/j.bcmd.2021.102544. Epub 2021 Feb 3. Blood Cells Mol Dis. 2021. PMID: 33610115
-
Experience with combination of hydroxyurea and low-dose thalidomide in transfusion-dependent beta thalassemia patients.Ann Hematol. 2021 Jun;100(6):1417-1427. doi: 10.1007/s00277-021-04501-3. Epub 2021 Apr 3. Ann Hematol. 2021. PMID: 33811502
-
Thalidomide and Hydroxyurea in Transfusion-Dependent Thalassemia: Efficacy, Safety Profile and Impact on Quality of Life.Hemoglobin. 2024 May;48(3):161-168. doi: 10.1080/03630269.2024.2386076. Epub 2024 Aug 2. Hemoglobin. 2024. PMID: 39092801 Clinical Trial.
-
Investigating the Efficacy and Safety of Thalidomide for Treating Patients With ß-Thalassemia: A Meta-Analysis.Front Pharmacol. 2022 Jan 11;12:814302. doi: 10.3389/fphar.2021.814302. eCollection 2021. Front Pharmacol. 2022. PMID: 35087410 Free PMC article. Review.
-
Hydroxyurea for lifelong transfusion-dependent β-thalassemia: A meta-analysis.Pediatr Hematol Oncol. 2017 Nov;34(8):435-448. doi: 10.1080/08880018.2017.1354948. Epub 2018 Jan 16. Pediatr Hematol Oncol. 2017. PMID: 29337597 Review.
Cited by
-
A cellular reporter system to evaluate endogenous fetal hemoglobin induction and screen for therapeutic compounds.Hemasphere. 2024 Aug 6;8(8):e139. doi: 10.1002/hem3.139. eCollection 2024 Aug. Hemasphere. 2024. PMID: 39108322 Free PMC article.
-
Efficacy and safety of thalidomide with hydroxyurea in sickle cell anemia: a quasi-experimental clinical trial.Blood Res. 2025 Apr 1;60(1):21. doi: 10.1007/s44313-025-00068-4. Blood Res. 2025. PMID: 40167946 Free PMC article.
-
Thalassemia in Bangladesh: progress, challenges, and a strategic blueprint for prevention.Orphanet J Rare Dis. 2025 Jul 10;20(1):358. doi: 10.1186/s13023-025-03744-x. Orphanet J Rare Dis. 2025. PMID: 40640823 Free PMC article. Review.
-
Comparison of Efficacy and Safety Outcomes of Different Doses Schedules of Thalidomide for Treating Moderate-to-Severe β-Thalassemia Patients.Ther Clin Risk Manag. 2024 Nov 29;20:799-809. doi: 10.2147/TCRM.S481128. eCollection 2024. Ther Clin Risk Manag. 2024. PMID: 39633983 Free PMC article.
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

