A stable and replicable neural signature of lifespan adversity in the adult brain
- PMID: 37604888
- PMCID: PMC10471497
- DOI: 10.1038/s41593-023-01410-8
A stable and replicable neural signature of lifespan adversity in the adult brain
Abstract
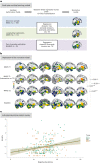
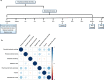
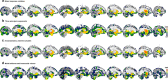
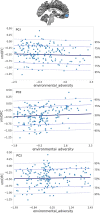
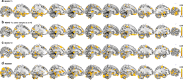
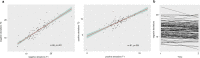
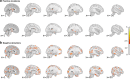
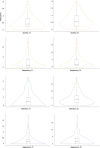
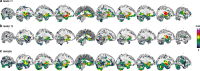
Environmental adversities constitute potent risk factors for psychiatric disorders. Evidence suggests the brain adapts to adversity, possibly in an adversity-type and region-specific manner. However, the long-term effects of adversity on brain structure and the association of individual neurobiological heterogeneity with behavior have yet to be elucidated. Here we estimated normative models of structural brain development based on a lifespan adversity profile in a longitudinal at-risk cohort aged 25 years (n = 169). This revealed widespread morphometric changes in the brain, with partially adversity-specific features. This pattern was replicated at the age of 33 years (n = 114) and in an independent sample at 22 years (n = 115). At the individual level, greater volume contractions relative to the model were predictive of future anxiety. We show a stable neurobiological signature of adversity that persists into adulthood and emphasize the importance of considering individual-level rather than group-level predictions to explain emerging psychopathology.
© 2023. The Author(s).
Conflict of interest statement
T.B. served in an advisory or consultancy role for eye level, Infectopharm, Lundbeck, Medice, Neurim Pharmaceuticals, Oberberg GmbH, Roche and Takeda. He received conference support or speaker’s fee from Janssen, Medice and Takeda. L.P. served in an advisory or consultancy role for Roche and Viforpharm and received speaker’s fee from Shire. She received royalties from Hogrefe, Kohlhammer and Schattauer. C.F.B. is cofounder and director of SBGneuro. The present work is unrelated to the above grants and relationships. The other authors report no potential conflicts of interest.
Figures




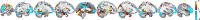
Similar articles
-
Lifespan adversities affect neural correlates of behavioral inhibition in adults.Front Psychiatry. 2024 Jan 16;15:1298695. doi: 10.3389/fpsyt.2024.1298695. eCollection 2024. Front Psychiatry. 2024. PMID: 38317765 Free PMC article.
-
The impact of adversities across the lifespan on psychological symptom profiles in late adulthood: a latent profile analysis.J Dev Orig Health Dis. 2023 Aug;14(4):508-522. doi: 10.1017/S2040174423000181. Epub 2023 Jul 21. J Dev Orig Health Dis. 2023. PMID: 37477375
-
Association between childhood adversities and psychopathology onset throughout the lifespan: Findings from a large metropolitan population.J Psychiatr Res. 2021 Mar;135:8-14. doi: 10.1016/j.jpsychires.2020.12.046. Epub 2020 Dec 19. J Psychiatr Res. 2021. PMID: 33406459
-
The link between child abuse and psychopathology: a review of neurobiological and genetic research.J R Soc Med. 2012 Apr;105(4):151-6. doi: 10.1258/jrsm.2011.110222. J R Soc Med. 2012. PMID: 22532655 Free PMC article. Review.
-
Research review: the neurobiology and genetics of maltreatment and adversity.J Child Psychol Psychiatry. 2010 Oct;51(10):1079-95. doi: 10.1111/j.1469-7610.2010.02271.x. J Child Psychol Psychiatry. 2010. PMID: 20546078 Review.
Cited by
-
Semantic search helper: A tool based on the use of embeddings in multi-item questionnaires as a harmonization opportunity for merging large datasets - A feasibility study.Eur Psychiatry. 2025 Jan 20;68(1):e8. doi: 10.1192/j.eurpsy.2024.1808. Eur Psychiatry. 2025. PMID: 39831376 Free PMC article.
-
Dissecting task-based fMRI activity using normative modelling: an application to the Emotional Face Matching Task.Commun Biol. 2024 Jul 20;7(1):888. doi: 10.1038/s42003-024-06573-z. Commun Biol. 2024. PMID: 39033247 Free PMC article.
-
A multimodal neural signature of face processing in autism within the fusiform gyrus.Nat Ment Health. 2025;3(1):31-45. doi: 10.1038/s44220-024-00349-4. Epub 2025 Jan 2. Nat Ment Health. 2025. PMID: 39802935 Free PMC article.
-
Lifespan adversities affect neural correlates of behavioral inhibition in adults.Front Psychiatry. 2024 Jan 16;15:1298695. doi: 10.3389/fpsyt.2024.1298695. eCollection 2024. Front Psychiatry. 2024. PMID: 38317765 Free PMC article.
-
Stress and substance use disorders: risk, relapse, and treatment outcomes.J Clin Invest. 2024 Aug 15;134(16):e172883. doi: 10.1172/JCI172883. J Clin Invest. 2024. PMID: 39145454 Free PMC article. Review.
References
-
- McCrory EJ, Viding E. The theory of latent vulnerability: reconceptualizing the link between childhood maltreatment and psychiatric disorder. Dev. Psychopathol. 2015;27:493–505. - PubMed
-
- Holz NE, et al. Early social adversity, altered brain functional connectivity, and mental health. Biol. Psychiatry. 2023;93:430–441. - PubMed
-
- Holz NE, Tost H, Meyer-Lindenberg A. Resilience and the brain: a key role for regulatory circuits linked to social stress and support. Mol. Psychiatry. 2020;25:379–396. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

