Insights into cellular behavior and micromolecular communication in urothelial micrografts
- PMID: 37604899
- PMCID: PMC10442416
- DOI: 10.1038/s41598-023-40049-0
Insights into cellular behavior and micromolecular communication in urothelial micrografts
Abstract
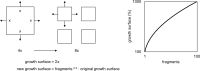
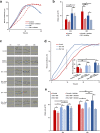
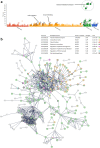
Autologous micrografting is a technique currently applied within skin wound healing, however, the potential use for surgical correction of other organs with epithelial lining, including the urinary bladder, remains largely unexplored. Currently, little is known about the micrograft expansion potential and the micromolecular events that occur in micrografted urothelial cells. In this study, we aimed to evaluate the proliferative potential of different porcine urothelial micrograft sizes in vitro, and, furthermore, to explore how urothelial micrografts communicate and which microcellular events are triggered. We demonstrated that increased tissue fragmentation subsequently potentiated the yield of proliferative cells and the cellular expansion potential, which confirms, that the micrografting principles of skin epithelium also apply to uroepithelium. Furthermore, we targeted the expression of the extracellular signal-regulated kinase (ERK) pathway and demonstrated that ERK activation occurred predominately at the micrograft borders and that ERK inhibition led to decreased urothelial migration and proliferation. Finally, we successfully isolated extracellular vesicles from the micrograft culture medium and evaluated their contents and relevance within various enriched biological processes. Our findings substantiate the potential of applying urothelial micrografting in future tissue-engineering models for reconstructive urological surgery, and, furthermore, highlights certain mechanisms as potential targets for future wound healing treatments.
© 2023. Springer Nature Limited.
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Cranidis A, Nestoridis G. Bladder augmentation. Int. Urogynecol. J. Pelvic Floor Dysfunct. 2000;11:33–40. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

