Targeting SphK1/2 by SKI-178 inhibits prostate cancer cell growth
- PMID: 37604912
- PMCID: PMC10442381
- DOI: 10.1038/s41419-023-06023-4
Targeting SphK1/2 by SKI-178 inhibits prostate cancer cell growth
Abstract
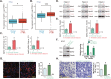
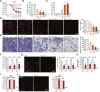
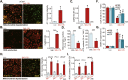
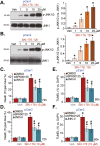
Sphingosine kinases (SphK), including SphK1 and SphK2, are important enzymes promoting progression of prostate cancer. SKI-178 is a novel and highly potent SphK1/2 dual inhibitor. We here tested the potential anti-prostate cancer cell activity of SKI-178. Bioinformatics analyses and results from local tissues demonstrated that that both SphK1 and SphK2 are upregulated in human prostate cancer tissues. Ectopic overexpression of SphK1 and SphK2, by lentiviral constructs, promoted primary prostate cancer cell proliferation and migration. In primary human prostate cancer cells and immortalized cell lines, SKI-178 potently inhibited cell viability, proliferation, cell cycle progression and cell migration, causing robust cell death and apoptosis. SKI-178 impaired mitochondrial functions, causing mitochondrial depolarization, reactive oxygen species production and ATP depletion.SKI-178 potently inhibited SphK activity and induced ceramide production, without affecting SphK1/2 expression in prostate cancer cells. Further, SKI-178 inhibited Akt-mTOR activation and induced JNK activation in prostate cancer cells. Contrarily, a constitutively-active Akt1 construct or the pharmacological JNK inhibitors attenuated SKI-178-induced cytotoxicity in prostate cancer cells. In vivo, daily intraperitoneal injection of a single dose of SKI-178 potently inhibited PC-3 xenograft growth in nude mice. SphK inhibition, ceramide production, ATP depletion and lipid peroxidation as well as Akt-mTOR inactivation and JNK activation were detected in PC-3 xenograft tissues with SKI-178 administration. Together, targeting SphK1/2 by SKI-178 potently inhibited prostate cancer cell growth in vitro and in vivo.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures




Similar articles
-
Targeting sphingosine kinase 1/2 by a novel dual inhibitor SKI-349 suppresses non-small cell lung cancer cell growth.Cell Death Dis. 2022 Jul 12;13(7):602. doi: 10.1038/s41419-022-05049-4. Cell Death Dis. 2022. PMID: 35831279 Free PMC article.
-
The sphingosine kinase inhibitor SKI-V suppresses cervical cancer cell growth.Int J Biol Sci. 2022 Apr 18;18(7):2994-3005. doi: 10.7150/ijbs.71381. eCollection 2022. Int J Biol Sci. 2022. PMID: 35541904 Free PMC article.
-
The anti-osteosarcoma cell activity by the sphingosine kinase 1 inhibitor SKI-V.Cell Death Discov. 2022 Feb 3;8(1):48. doi: 10.1038/s41420-022-00838-4. Cell Death Discov. 2022. PMID: 35115496 Free PMC article.
-
GNE-493 inhibits prostate cancer cell growth via Akt-mTOR-dependent and -independent mechanisms.Cell Death Discov. 2022 Mar 16;8(1):120. doi: 10.1038/s41420-022-00911-y. Cell Death Discov. 2022. PMID: 35296639 Free PMC article.
-
GDC-0349 inhibits non-small cell lung cancer cell growth.Cell Death Dis. 2020 Nov 5;11(11):951. doi: 10.1038/s41419-020-03146-w. Cell Death Dis. 2020. PMID: 33154352 Free PMC article.
Cited by
-
Exploring Autophagy-Related Gene Expression in Hepatocellular Carcinoma via TCGA, GEPIA2, and HPA Databases: Implications for Prognosis.J Hepatocell Carcinoma. 2025 Jul 22;12:1557-1586. doi: 10.2147/JHC.S520917. eCollection 2025. J Hepatocell Carcinoma. 2025. PMID: 40726620 Free PMC article.
-
SPHK1 enhances olaparib resistance in ovarian cancer through the NFκB/NRF2/ferroptosis pathway.Cell Death Discov. 2025 Jan 28;11(1):29. doi: 10.1038/s41420-025-02309-y. Cell Death Discov. 2025. PMID: 39875359 Free PMC article.
-
OTUB1 antagonizes TRIM21 to induce deubiquitination of SPHK1 and promote the progression of hepatocellular carcinoma.Oncogene. 2025 Aug 30. doi: 10.1038/s41388-025-03556-0. Online ahead of print. Oncogene. 2025. PMID: 40885853
-
SPHK1 promotes HNSCC immune evasion by regulating the MMP1-PD-L1 axis.Theranostics. 2024 Oct 28;14(18):7199-7218. doi: 10.7150/thno.102390. eCollection 2024. Theranostics. 2024. PMID: 39629135 Free PMC article.
-
[Sphingosine kinase-1 regulates migration and invasion of gastric cancer cells via targeting the nuclear factor-κB signaling pathway].Nan Fang Yi Ke Da Xue Xue Bao. 2024 Nov 20;44(11):2163-2171. doi: 10.12122/j.issn.1673-4254.2024.11.13. Nan Fang Yi Ke Da Xue Xue Bao. 2024. PMID: 39623272 Free PMC article. Chinese.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

