Amyloid-β slows cilia movement along the ventricle, impairs fluid flow, and exacerbates its neurotoxicity in explant culture
- PMID: 37605005
- PMCID: PMC10442439
- DOI: 10.1038/s41598-023-40742-0
Amyloid-β slows cilia movement along the ventricle, impairs fluid flow, and exacerbates its neurotoxicity in explant culture
Abstract
Alzheimer's disease (AD) is characterized by extensive and selective death of neurons and deterioration of synapses and circuits in the brain. The Aβ1-42 concentration is higher in an AD brain than in cognitively normal elderly individuals, and Aβ1-42 exhibits neurotoxicity. Brain-derived Aβ is transported into the cerebrospinal fluid (CSF), and CSF flow is driven in part by the beating of cilia and CSF secretion into ventricles. Ventricles are lined with ependyma whose apical surface is covered with motile cilia. Herein, we constructed an experimental system to measure the movement of ependymal cilia and examined the effects of Aβ1-42 to the beating of cilia and neurons. The circadian rhythm of the beating frequency of ependymal cilia was detected using brain wall explant-cultures containing ependymal cilia and neurons; the beating frequency was high at midday and low at midnight. Aβ1-42 decreased the peak frequency of ciliary beating at midday and slightly increased it at midnight. Aβ1-42 exhibited neurotoxicity to neurons on the non-ciliated side of the explant culture, while the neurotoxicity was less evident in neurons on the ciliated side. The neurotoxic effect of Aβ1-42 was diminished when 1 mPa of shear stress was generated using a flow chamber system that mimicked the flow by cilia. These results indicate that Aβ1-42 affects the circadian rhythm of ciliary beating, decreases the medium flow by the cilia-beating, and enhances the neurotoxic action of Aβ1-42 in the brain explant culture.
© 2023. Springer Nature Limited.
Conflict of interest statement
The authors declare no competing interests.
Figures

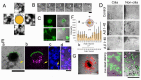

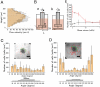
Similar articles
-
Ciliary Beating Compartmentalizes Cerebrospinal Fluid Flow in the Brain and Regulates Ventricular Development.Curr Biol. 2019 Jan 21;29(2):229-241.e6. doi: 10.1016/j.cub.2018.11.059. Epub 2019 Jan 3. Curr Biol. 2019. PMID: 30612902 Free PMC article.
-
The organic mercury compounds, methylmercury and ethylmercury, inhibited ciliary movement of ventricular ependymal cells in the mouse brain around the concentrations reported for human poisoning.Neurotoxicology. 2016 Dec;57:69-74. doi: 10.1016/j.neuro.2016.08.007. Epub 2016 Sep 13. Neurotoxicology. 2016. PMID: 27620881
-
Alcohol consumption impairs the ependymal cilia motility in the brain ventricles.Sci Rep. 2017 Oct 20;7(1):13652. doi: 10.1038/s41598-017-13947-3. Sci Rep. 2017. PMID: 29057897 Free PMC article.
-
Exploring mechanisms of ventricular enlargement in idiopathic normal pressure hydrocephalus: a role of cerebrospinal fluid dynamics and motile cilia.Fluids Barriers CNS. 2021 Apr 19;18(1):20. doi: 10.1186/s12987-021-00243-6. Fluids Barriers CNS. 2021. PMID: 33874972 Free PMC article. Review.
-
Planar polarity of ependymal cilia.Differentiation. 2012 Feb;83(2):S86-90. doi: 10.1016/j.diff.2011.10.007. Epub 2011 Nov 17. Differentiation. 2012. PMID: 22101065 Review.
Cited by
-
Centrosomes and cilia in neurodegeneration: main actors or mere spectators?Open Biol. 2025 May;15(5):240317. doi: 10.1098/rsob.240317. Epub 2025 May 21. Open Biol. 2025. PMID: 40393509 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

