Mitochondrial impairment and synaptic dysfunction are associated with neurological defects in iPSCs-derived cortical neurons of MERRF patients
- PMID: 37605213
- PMCID: PMC10441704
- DOI: 10.1186/s12929-023-00966-8
Mitochondrial impairment and synaptic dysfunction are associated with neurological defects in iPSCs-derived cortical neurons of MERRF patients
Abstract
Background: Myoclonic epilepsy with ragged-red fibers (MERRF) syndrome is a rare inherited mitochondrial disease mainly caused by the m.8344A > G mutation in mitochondrial tRNALys gene, and usually manifested as complex neurological disorders and muscle weakness. Currently, the pathogenic mechanism of this disease has not yet been resolved, and there is no effective therapy for MERRF syndrome. In this study, MERRF patients-derived iPSCs were used to model patient-specific neurons for investigation of the pathogenic mechanism of neurological disorders in mitochondrial disease.
Methods: MERRF patient-derived iPSCs were differentiated into excitatory glutamatergic neurons to unravel the effects of the m.8344A > G mutation on mitochondrial bioenergetic function, neural-lineage differentiation and neuronal function. By the well-established differentiation protocol and electrophysiological activity assay platform, we examined the pathophysiological behaviors in cortical neurons of MERRF patients.
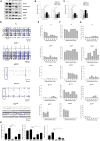
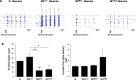
Results: We have successfully established the iPSCs-derived neural progenitor cells and cortical-like neurons of patients with MERRF syndrome that retained the heteroplasmy of the m.8344A > G mutation from the patients' skin fibroblasts and exhibited the phenotype of the mitochondrial disease. MERRF neural cells harboring the m.8344A > G mutation exhibited impaired mitochondrial bioenergetic function, elevated ROS levels and imbalanced expression of antioxidant enzymes. Our findings indicate that neural immaturity and synaptic protein loss led to the impairment of neuronal activity and plasticity in MERRF neurons harboring the m.8344A > G mutation. By electrophysiological recordings, we monitored the in vivo neuronal behaviors of MERRF neurons and found that neurons harboring a high level of the m.8344A > G mutation exhibited impairment of the spontaneous and evoked potential-stimulated neuronal activities.
Conclusions: We demonstrated for the first time the link of mitochondrial impairment and synaptic dysfunction to neurological defects through impeding synaptic plasticity in excitatory neurons derived from iPSCs of MERRF patients harboring the m.8344A > G mutation. This study has provided new insight into the pathogenic mechanism of the tRNALys gene mutation of mtDNA, which is useful for the development of a patient-specific iPSCs platform for disease modeling and screening of new drugs to treat patients with MERRF syndrome.
Keywords: AMPARs; Disease modeling; Electrophysiological activity; Excitatory neurons; MERRF syndrome; Neurological defect; Synaptic plasticity; Synaptophysin; iPSCs; mtDNA mutation.
© 2023. National Science Council of the Republic of China (Taiwan).
Conflict of interest statement
The authors declare no competing interests.
Figures





Similar articles
-
Distal weakness with respiratory insufficiency caused by the m.8344A > G "MERRF" mutation.Neuromuscul Disord. 2014 Jun;24(6):533-6. doi: 10.1016/j.nmd.2014.03.011. Epub 2014 Apr 1. Neuromuscul Disord. 2014. PMID: 24792523 Free PMC article.
-
Rapamycin rescues mitochondrial dysfunction in cells carrying the m.8344A > G mutation in the mitochondrial tRNALys.Mol Med. 2022 Aug 3;28(1):90. doi: 10.1186/s10020-022-00519-z. Mol Med. 2022. PMID: 35922766 Free PMC article.
-
Identification of new variants in MTRNR1 and MTRNR2 genes using whole mitochondrial genome sequencing in a Taiwanese family with MERRF (myoclonic epilepsy with ragged-red fibers) syndrome.Hear Res. 2023 Oct;438:108876. doi: 10.1016/j.heares.2023.108876. Epub 2023 Aug 22. Hear Res. 2023. PMID: 37683310 Review.
-
Expanded phenotypic spectrum of the m.8344A>G "MERRF" mutation: data from the German mitoNET registry.J Neurol. 2016 May;263(5):961-972. doi: 10.1007/s00415-016-8086-3. Epub 2016 Mar 19. J Neurol. 2016. PMID: 26995359
-
Mitochondrial DNA mutation-elicited oxidative stress, oxidative damage, and altered gene expression in cultured cells of patients with MERRF syndrome.Mol Neurobiol. 2010 Jun;41(2-3):256-66. doi: 10.1007/s12035-010-8123-7. Epub 2010 Apr 23. Mol Neurobiol. 2010. PMID: 20411357 Review.
Cited by
-
Mitochondrial diseases: from molecular mechanisms to therapeutic advances.Signal Transduct Target Ther. 2025 Jan 10;10(1):9. doi: 10.1038/s41392-024-02044-3. Signal Transduct Target Ther. 2025. PMID: 39788934 Free PMC article. Review.
-
Reactive oxygen species regulate adipose-osteogenic lineage commitment of human mesenchymal stem cells by modulating gene expression of C/EBP homology protein and aldo-keto reductase family 1 member A1.Cell Biosci. 2025 Jul 18;15(1):104. doi: 10.1186/s13578-025-01448-0. Cell Biosci. 2025. PMID: 40682073 Free PMC article.
-
Current Applications of Human Pluripotent Stem Cells in Neuroscience Research and Cell Transplantation Therapy for Neurological Disorders.Stem Cell Rev Rep. 2025 May;21(4):964-987. doi: 10.1007/s12015-025-10851-6. Epub 2025 Apr 5. Stem Cell Rev Rep. 2025. PMID: 40186708 Review.
-
Mitochondrial Transplantation/Transfer: Promising Therapeutic Strategies for Spinal Cord Injury.J Orthop Translat. 2025 May 16;52:441-450. doi: 10.1016/j.jot.2025.04.017. eCollection 2025 May. J Orthop Translat. 2025. PMID: 40485848 Free PMC article. Review.
-
Clinical Approaches for Mitochondrial Diseases.Cells. 2023 Oct 20;12(20):2494. doi: 10.3390/cells12202494. Cells. 2023. PMID: 37887337 Free PMC article. Review.
References
-
- Russell OM, Gorman GS, Lightowlers RN, Turnbull DM. Mitochondrial diseases: hope for the future. Cell. 2020;181:168–188. - PubMed
-
- Lorenzoni PJ, Scola RH, Kay CS, Arndt RC, Silvado CE, Werneck LC. MERRF: clinical features, muscle biopsy and molecular genetics in Brazilian patients. Mitochondrion. 2011;11:528–532. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

