Current approaches to develop "off-the-shelf" chimeric antigen receptor (CAR)-T cells for cancer treatment: a systematic review
- PMID: 37605218
- PMCID: PMC10440917
- DOI: 10.1186/s40164-023-00435-w
Current approaches to develop "off-the-shelf" chimeric antigen receptor (CAR)-T cells for cancer treatment: a systematic review
Abstract
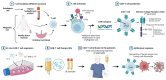
Chimeric antigen receptor (CAR)-T cell therapy is one of the most promising advances in cancer treatment. It is based on genetically modified T cells to express a CAR, which enables the recognition of the specific tumour antigen of interest. To date, CAR-T cell therapies approved for commercialisation are designed to treat haematological malignancies, showing impressive clinical efficacy in patients with relapsed or refractory advanced-stage tumours. However, since they all use the patient´s own T cells as starting material (i.e. autologous use), they have important limitations, including manufacturing delays, high production costs, difficulties in standardising the preparation process, and production failures due to patient T cell dysfunction. Therefore, many efforts are currently being devoted to contribute to the development of safe and effective therapies for allogeneic use, which should be designed to overcome the most important risks they entail: immune rejection and graft-versus-host disease (GvHD). This systematic review brings together the wide range of different approaches that have been studied to achieve the production of allogeneic CAR-T cell therapies and discuss the advantages and disadvantages of every strategy. The methods were classified in two major categories: those involving extra genetic modifications, in addition to CAR integration, and those relying on the selection of alternative cell sources/subpopulations for allogeneic CAR-T cell production (i.e. γδ T cells, induced pluripotent stem cells (iPSCs), umbilical cord blood T cells, memory T cells subpopulations, virus-specific T cells and cytokine-induced killer cells). We have observed that, although genetic modification of T cells is the most widely used approach, new approaches combining both methods have emerged. However, more preclinical and clinical research is needed to determine the most appropriate strategy to bring this promising antitumour therapy to the clinical setting.
Keywords: Advanced therapy medicinal products (ATMPs); Allogeneic treatment; Allorejection; Cancer immunotherapy; Chimeric antigen receptor (CAR)-T cells; Genetic engineering; Graft-versus-host disease (GvHD); Systematic review; “Off-the-shelf” adoptive T cell therapy.
© 2023. YUMED Inc. and BioMed Central Ltd.
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Allogeneic "Off-the-Shelf" CAR T cells: Challenges and advances.Best Pract Res Clin Haematol. 2024 Sep;37(3):101566. doi: 10.1016/j.beha.2024.101566. Epub 2024 Jul 25. Best Pract Res Clin Haematol. 2024. PMID: 39396256 Review.
-
The paths and challenges of "off-the-shelf" CAR-T cell therapy: An overview of clinical trials.Biomed Pharmacother. 2023 Dec 31;169:115888. doi: 10.1016/j.biopha.2023.115888. Epub 2023 Nov 17. Biomed Pharmacother. 2023. PMID: 37979380 Review.
-
Potential alternatives to αβ-T cells to prevent graft-versus-host disease (GvHD) in allogeneic chimeric antigen receptor (CAR)-based cancer immunotherapy: A comprehensive review.Pathol Res Pract. 2024 Oct;262:155518. doi: 10.1016/j.prp.2024.155518. Epub 2024 Aug 10. Pathol Res Pract. 2024. PMID: 39146830 Review.
-
Chimeric antigen receptor (CAR) natural killer (NK)-cell therapy: leveraging the power of innate immunity.Br J Haematol. 2021 Apr;193(2):216-230. doi: 10.1111/bjh.17186. Epub 2020 Nov 20. Br J Haematol. 2021. PMID: 33216984 Free PMC article. Review.
-
Strategies for overcoming bottlenecks in allogeneic CAR-T cell therapy.Front Immunol. 2023 Jul 24;14:1199145. doi: 10.3389/fimmu.2023.1199145. eCollection 2023. Front Immunol. 2023. PMID: 37554322 Free PMC article. Review.
Cited by
-
Global research trends in CAR-T cell therapy for solid tumors: A comprehensive visualization and bibliometric study (2012-2023).Hum Vaccin Immunother. 2024 Dec 31;20(1):2338984. doi: 10.1080/21645515.2024.2338984. Epub 2024 May 2. Hum Vaccin Immunother. 2024. PMID: 38698555 Free PMC article.
-
CAR T Cell Nanosymbionts: Revealing the Boundless Potential of a New Dyad.Int J Mol Sci. 2024 Dec 7;25(23):13157. doi: 10.3390/ijms252313157. Int J Mol Sci. 2024. PMID: 39684867 Free PMC article. Review.
-
Enhancing precision in cancer treatment: the role of gene therapy and immune modulation in oncology.Front Med (Lausanne). 2025 Jan 13;11:1527600. doi: 10.3389/fmed.2024.1527600. eCollection 2024. Front Med (Lausanne). 2025. PMID: 39871848 Free PMC article. Review.
-
Chimeric antigen receptor T-cell therapy in childhood acute myeloid leukemia: how far are we from a clinical application?Haematologica. 2024 Jun 1;109(6):1656-1667. doi: 10.3324/haematol.2023.283817. Haematologica. 2024. PMID: 38832421 Free PMC article. Review.
-
CD7 CAR-T therapy: current developments, improvements, and dilemmas.Blood Sci. 2025 Aug 5;7(3):e00247. doi: 10.1097/BS9.0000000000000247. eCollection 2025 Sep. Blood Sci. 2025. PMID: 40771729 Free PMC article. Review.
References
-
- KYMRIAH (tisagenlecleucel) | FDA. 2022. https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-produ.... Accessed 13 Oct 2022.
-
- Kymriah | European Medicines Agency. 2022. https://www.ema.europa.eu/en/medicines/human/EPAR/kymriah. Accessed 21 Dec 2022.
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

