Bioengineered skin organoids: from development to applications
- PMID: 37605220
- PMCID: PMC10463602
- DOI: 10.1186/s40779-023-00475-7
Bioengineered skin organoids: from development to applications
Abstract
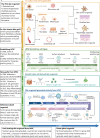
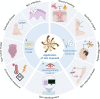
Significant advancements have been made in recent years in the development of highly sophisticated skin organoids. Serving as three-dimensional models that mimic human skin, these organoids have evolved into complex structures and are increasingly recognized as effective alternatives to traditional culture models and human skin due to their ability to overcome the limitations of two-dimensional systems and ethical concerns. The inherent plasticity of skin organoids allows for their construction into physiological and pathological models, enabling the study of skin development and dynamic changes. This review provides an overview of the pivotal work in the progression from 3D layered epidermis to cyst-like skin organoids with appendages. Furthermore, it highlights the latest advancements in organoid construction facilitated by state-of-the-art engineering techniques, such as 3D printing and microfluidic devices. The review also summarizes and discusses the diverse applications of skin organoids in developmental biology, disease modelling, regenerative medicine, and personalized medicine, while considering their prospects and limitations.
Keywords: Disease modelling; Organoid generation; Regenerative medicine; Skin appendage; Skin organoid; Tissue engineering.
© 2023. People´s Military Medical Press.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
Insights on Three Dimensional Organoid Studies for Stem Cell Therapy in Regenerative Medicine.Stem Cell Rev Rep. 2024 Feb;20(2):509-523. doi: 10.1007/s12015-023-10655-6. Epub 2023 Dec 14. Stem Cell Rev Rep. 2024. PMID: 38095787 Free PMC article. Review.
-
Current strategies using 3D organoids to establish in vitro maternal-embryonic interaction.J Vet Sci. 2024 May;25(3):e40. doi: 10.4142/jvs.24004. J Vet Sci. 2024. PMID: 38834510 Free PMC article. Review.
-
Challenges in Bio-fabrication of Organoid Cultures.Adv Exp Med Biol. 2018;1107:53-71. doi: 10.1007/5584_2018_216. Adv Exp Med Biol. 2018. PMID: 29855825 Review.
-
Sebaceous gland organoid engineering.Burns Trauma. 2024 May 1;12:tkae003. doi: 10.1093/burnst/tkae003. eCollection 2024. Burns Trauma. 2024. PMID: 38699464 Free PMC article. Review.
-
Organoid technology: Current standing and future perspectives.Stem Cells. 2021 Dec;39(12):1625-1649. doi: 10.1002/stem.3379. Epub 2021 Sep 30. Stem Cells. 2021. PMID: 33786925 Review.
Cited by
-
Advancements in 3D skin bioprinting: processes, bioinks, applications and sensor integration.Int J Extrem Manuf. 2025 Feb 1;7(1):012009. doi: 10.1088/2631-7990/ad878c. Epub 2024 Nov 19. Int J Extrem Manuf. 2025. PMID: 39569402 Free PMC article. Review.
-
Accelerating cartilage regeneration with DNA-SF hydrogel sustained release system-based cartilage organoids.Mil Med Res. 2025 Jul 28;12(1):39. doi: 10.1186/s40779-025-00625-z. Mil Med Res. 2025. PMID: 40722047 Free PMC article.
-
Applications of Engineered Skin Tissue for Cosmetic Component and Toxicology Detection.Cell Transplant. 2024 Jan-Dec;33:9636897241235464. doi: 10.1177/09636897241235464. Cell Transplant. 2024. PMID: 38491929 Free PMC article. Review.
-
Curcumin-Loaded Nanocomposite Hydrogel Dressings for Promoting Infected Wound Healing and Tissue Regeneration.Int J Nanomedicine. 2024 Oct 18;19:10479-10496. doi: 10.2147/IJN.S479330. eCollection 2024. Int J Nanomedicine. 2024. PMID: 39439502 Free PMC article.
-
Biomaterial-based mechanical regulation facilitates scarless wound healing with functional skin appendage regeneration.Mil Med Res. 2024 Feb 18;11(1):13. doi: 10.1186/s40779-024-00519-6. Mil Med Res. 2024. PMID: 38369464 Free PMC article. Review.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

