Ferroptosis of Microglia in Aging Human White Matter Injury
- PMID: 37605362
- PMCID: PMC10840747
- DOI: 10.1002/ana.26770
Ferroptosis of Microglia in Aging Human White Matter Injury
Abstract
Objective: Because the role of white matter (WM) degenerating microglia (DM) in remyelination failure is unclear, we sought to define the core features of this novel population of aging human microglia.
Methods: We analyzed postmortem human brain tissue to define a population of DM in aging WM lesions. We used immunofluorescence staining and gene expression analysis to investigate molecular mechanisms related to the degeneration of DM.
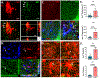
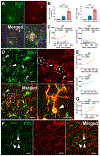
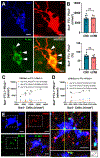
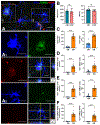
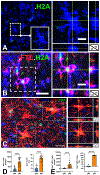
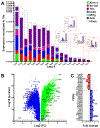
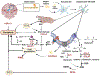
Results: We found that DM, which accumulated myelin debris were selectively enriched in the iron-binding protein light chain ferritin, and accumulated PLIN2-labeled lipid droplets. DM displayed lipid peroxidation injury and enhanced expression for TOM20, a mitochondrial translocase, and a sensor of oxidative stress. DM also displayed enhanced expression of the DNA fragmentation marker phospho-histone H2A.X. We identified a unique set of ferroptosis-related genes involving iron-mediated lipid dysmetabolism and oxidative stress that were preferentially expressed in WM injury relative to gray matter neurodegeneration.
Interpretation: Ferroptosis appears to be a major mechanism of WM injury in Alzheimer's disease and vascular dementia. WM DM are a novel therapeutic target to potentially reduce the impact of WM injury and myelin loss on the progression of cognitive impairment. ANN NEUROL 2023;94:1048-1066.
© 2023 The Authors. Annals of Neurology published by Wiley Periodicals LLC on behalf of American Neurological Association.
Conflict of interest statement
Figures
References
-
- Sam K, Crawley AP, Conklin J, et al. Development of white matter hyperintensity is preceded by reduced cerebrovascular reactivity. Ann Neurol 2016;80:277–285. - PubMed
Publication types
MeSH terms
Grants and funding
- AG065406/AG/NIA NIH HHS/United States
- AG054651/AG/NIA NIH HHS/United States
- AG031892/AG/NIA NIH HHS/United States
- R01 NS105984/NS/NINDS NIH HHS/United States
- R01 AG031892/AG/NIA NIH HHS/United States
- U01 AG006781/AG/NIA NIH HHS/United States
- p50 AG005136/AG/NIA NIH HHS/United States
- R01 AG054651/AG/NIA NIH HHS/United States
- U19 AG066567/AG/NIA NIH HHS/United States
- p30AG066509/AG/NIA NIH HHS/United States
- P50 AG005136/AG/NIA NIH HHS/United States
- NS105984/NS/NINDS NIH HHS/United States
- RF1 AG065406/AG/NIA NIH HHS/United States
- P30 AG066509/AG/NIA NIH HHS/United States
- R01 AG065406/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials

