Difference in respiratory syncytial virus-specific Fc-mediated antibody effector functions between children and adults
- PMID: 37605554
- PMCID: PMC10711356
- DOI: 10.1093/cei/uxad101
Difference in respiratory syncytial virus-specific Fc-mediated antibody effector functions between children and adults
Abstract
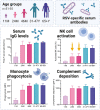
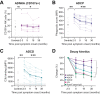
Respiratory syncytial virus (RSV) infections are a major cause of bronchiolitis and pneumonia in infants and older adults, for which there is no known correlate of protection. Increasing evidence suggests that Fc-mediated antibody effector functions have an important role, but little is known about the development, heterogeneity, and durability of these functional responses. In light of future vaccine strategies, a clear view of the immunological background and differences between various target populations is of crucial importance. In this study, we have assessed both quantitative and qualitative aspects of RSV-specific serum antibodies, including IgG/IgA levels, IgG subclasses, antibody-dependent complement deposition, cellular phagocytosis, and NK cell activation (ADNKA). Samples were collected cross-sectionally in different age groups (11-, 24-, and 46-month-old children, adults, and older adults; n = 31-35 per group) and longitudinally following natural RSV infection in (older) adults (2-36 months post-infection; n = 10). We found that serum of 24-month-old children induces significantly lower ADNKA than the serum of adults (P < 0.01), which is not explained by antibody levels. Furthermore, in (older) adults we observed boosting of antibody levels and functionality at 2-3 months after RSV infection, except for ADNKA. The strongest decrease was subsequently observed within the first 9 months, after which levels remained relatively stable up to three years post-infection. Together, these data provide a comprehensive overview of the functional landscape of RSV-specific serum antibodies in the human population, highlighting that while antibodies reach adult levels already at a young age, ADNKA requires more time to fully develop.
Keywords: anti-viral immunity; antibodies; complement; cytotoxicity; phagocytosis.
© The Author(s) 2023. Published by Oxford University Press on behalf of the British Society for Immunology.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures






References
-
- Shi T, Denouel A, Tietjen AK, Campbell I, Moran E, Li X, et al. . Global disease burden estimates of respiratory syncytial virus-associated acute respiratory infection in older adults in 2015: a systematic review and meta-analysis. J Infect Dis 2019,222 (Suppl 7), S577–S583. - PubMed
-
- Shi T, McAllister DA, O’Brien KL, Simoes EAF, Madhi SA, Gessner BD, et al. .; RSV Global Epidemiology Network. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: a systematic review and modelling study. Lancet 2017, 390, 946–58. doi:10.1016/S0140-6736(17)30938-8. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

