Three-dimensional spatial quantitative analysis of cardiac lymphatics in the mouse heart
- PMID: 37605603
- PMCID: PMC10592199
- DOI: 10.1111/micc.12826
Three-dimensional spatial quantitative analysis of cardiac lymphatics in the mouse heart
Abstract
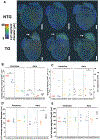
Objective: Three-dimensional (3D) microscopy and image data analysis are necessary for studying the morphology of cardiac lymphatic vessels (LyVs) and their association with other cell types. We aimed to develop a methodology for 3D multiplexed lightsheet microscopy and highly sensitive and quantitative image analysis to identify pathological remodeling in the 3D morphology of LyVs in young adult mouse hearts with familial hypertrophic cardiomyopathy (HCM).
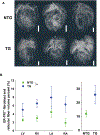
Methods: We developed a 3D lightsheet microscopy workflow providing a quick turn-around (as few as 5-6 days), multiplex fluorescence detection, and preservation of LyV structure and epitope markers. Hearts from non-transgenic and transgenic (TG) HCM mice were arrested in diastole, retrograde perfused, immunolabeled, optically cleared, and imaged. We built an image-processing pipeline to quantify LyV morphological parameters at the chamber and branch levels.
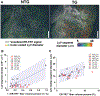
Results: Chamber-specific pathological alterations of LyVs were identified, and significant changes were seen in the right atrium (RA). TG hearts had a higher volume percent of ER-TR7+ fibroblasts and reticular fibers. In the RA, we found associations between ER-TR7+ volume percent and both LyV segment density and median diameter.
Conclusions: This workflow and study enabled multi-scale analysis of pathological changes in cardiac LyVs of young adult mice, inviting ideas for research on LyVs in cardiac disease.
Keywords: 3D imaging; cardiac lymphatics; multiplex fluorescence; optical tissue clearing; vessel morphology.
© 2023 The Authors. Microcirculation published by John Wiley & Sons Ltd.
Conflict of interest statement
Conflict of Interest: The authors declare no conflicts of interest.
Figures


Update of
-
Three-dimensional spatial quantitative analysis of cardiac lymphatics in the mouse heart.bioRxiv [Preprint]. 2023 Feb 3:2023.02.01.526338. doi: 10.1101/2023.02.01.526338. bioRxiv. 2023. Update in: Microcirculation. 2023 Oct;30(7):e12826. doi: 10.1111/micc.12826. PMID: 36778334 Free PMC article. Updated. Preprint.
References
-
- Johnson RA, and Blake TM (1966) Lymphatics of the heart. Circulation, 33 (1), 137–142. - PubMed
-
- Sacchi G, Weber E, Agliano M, Cavina N, and Comparini L (1999) Lymphatic vessels of the human heart: precollectors and collecting vessels. A morpho-structural study. J Submicrosc. Cytol. Pathol, 31 (4), 515–525. - PubMed
-
- Brakenhielm E, and Alitalo K (2019) Cardiac lymphatics in health and disease. Nature Reviews Cardiology, 16 (1), 56–68. - PubMed
-
- Brakenhielm E, González A, and Díez J (2020) Role of Cardiac Lymphatics in Myocardial Edema and Fibrosis: JACC Review Topic of the Week. Journal of the American College of Cardiology, 76 (6), 735–744. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

