Protein phosphatase 4 dephosphorylates phosphofructokinase-1 to regulate its enzymatic activity
- PMID: 37605615
- PMCID: PMC10689085
- DOI: 10.5483/BMBRep.2023-0065
Protein phosphatase 4 dephosphorylates phosphofructokinase-1 to regulate its enzymatic activity
Abstract
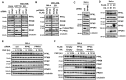
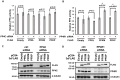
Most cancer cells utilize glucose at a high rate to produce energyand precursors for the biosynthesis of macromolecules such as lipids, proteins, and nucleic acids. This phenomenon is called the Warburg effect or aerobic glycolysis- this distinct characteristic is an attractive target for developing anticancer drugs. Here, we found that Phosphofructokinase-1 (PFK-1) is a substrate of the Protein Phosphatase 4 catalytic subunit (PP4C)/PP4 regulatory subunit 1 (PP4R1) complex by using immunoprecipitation and in vitro assay. While manipulation of PP4C/PP4R1 does not have a critical impact on PFK-1 expression, the absence of the PP4C/PP4R1 complex increases PFK-1 activity. Although PP4C depletion or overexpression does not cause a dramatic change in the overall glycolytic rate, PP4R1 depletion induces a considerable increase in both basal and compensatory glycolytic rates, as well as the oxygen consumption rate, indicating oxidative phosphorylation. Collectively, the PP4C/PP4R1 complex regulates PFK-1 activity by reversing its phosphorylation and is a promising candidate for treating glycolytic disorders and cancers. Targeting PP4R1 could be a more efficient and safer strategy to avoid pleiotropic effects than targeting PP4C directly. [BMB Reports 2023; 56(11): 618-623].
Conflict of interest statement
The authors have no conflicting interests.
Figures


Similar articles
-
Purification and identification of a novel subunit of protein serine/threonine phosphatase 4.J Biol Chem. 1999 Feb 26;274(9):5339-47. doi: 10.1074/jbc.274.9.5339. J Biol Chem. 1999. PMID: 10026142
-
Targeting of the catalytic subunit of protein phosphatase-1 to the glycolytic enzyme phosphofructokinase.Biochemistry. 1997 Jul 8;36(27):8318-24. doi: 10.1021/bi962814r. Biochemistry. 1997. PMID: 9204878
-
Leucine Carboxyl Methyltransferase 1 (LCMT-1) Methylates Protein Phosphatase 4 (PP4) and Protein Phosphatase 6 (PP6) and Differentially Regulates the Stable Formation of Different PP4 Holoenzymes.J Biol Chem. 2016 Sep 30;291(40):21008-21019. doi: 10.1074/jbc.M116.739920. Epub 2016 Aug 9. J Biol Chem. 2016. PMID: 27507813 Free PMC article.
-
Revisited Metabolic Control and Reprogramming Cancers by Means of the Warburg Effect in Tumor Cells.Int J Mol Sci. 2022 Sep 2;23(17):10037. doi: 10.3390/ijms231710037. Int J Mol Sci. 2022. PMID: 36077431 Free PMC article. Review.
-
Studies of gene expression and activity of hexokinase, phosphofructokinase and glycogen synthase in human skeletal muscle in states of altered insulin-stimulated glucose metabolism.Dan Med Bull. 1999 Feb;46(1):13-34. Dan Med Bull. 1999. PMID: 10081651 Review.
Cited by
-
Transcriptional regulation and post-translational modifications in the glycolytic pathway for targeted cancer therapy.Acta Pharmacol Sin. 2024 Aug;45(8):1533-1555. doi: 10.1038/s41401-024-01264-1. Epub 2024 Apr 15. Acta Pharmacol Sin. 2024. PMID: 38622288 Free PMC article. Review.

