Increased Expression of Tight Junction Proteins and Blood-Brain Barrier Integrity in MCAO Rats Following Injection of miR-149-5p
- PMID: 37605737
- PMCID: PMC10440002
- DOI: 10.22088/IJMCM.BUMS.11.3.223
Increased Expression of Tight Junction Proteins and Blood-Brain Barrier Integrity in MCAO Rats Following Injection of miR-149-5p
Abstract
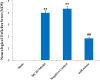
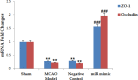
Cerebral ischemia is a common neurodegenerative disease in which damage to the blood-brain barrier (BBB) is the main consequence. In cerebral ischemia, the level of miR-149-5p and tight junction proteins are decreased, while the level of Calpine is increased, finally leading to increased BBB permeability. This study investigated the effect of miR-149-5p mimic on the expression of Calpain, Occludin, and ZO-1 and the consequences of cerebral ischemia. Cerebral ischemia model was performed via middle cerebral artery occlusion (MCAO) method on female Wistar rats. Four groups of Wistar rats were studied: Sham, cerebral ischemia without treatment, Scramble miR, and miR-149-5p mimic treatment. Then, neurological defects and BBB permeability (via Evans blue staining), cerebral edema (cerebrospinal fluid percentage), and ZO-1, Occludin, and Calapin expression (by quantitative real time- PCR) were investigated. qRT-PCR results showed miR-149-5p expression decreases after cerebral ischemia induction. In addition, Occludin and ZO-1 expression significantly increased in miR-149-5p group. In contrast, Calapin expression, BBB permeability, brain water content and neurological defects were significantly decreased. It seems that the increased level of miR-149-5p exerts its protective effect on cerebral ischemia due to increasing of tight junction proteins.
Keywords: Cerebral ischemia; miR-149-5p; middle cerebral artery occlusion; tight junction.
Figures
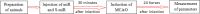




Similar articles
-
The neuroprotective effect of MicroRNA-149-5p and coenzymeQ10 by reducing levels of inflammatory cytokines and metalloproteinases following focal brain ischemia in rats.Brain Res Bull. 2021 Apr;169:205-213. doi: 10.1016/j.brainresbull.2021.01.013. Epub 2021 Jan 27. Brain Res Bull. 2021. PMID: 33508402
-
miR-671-5p Upregulation Attenuates Blood-Brain Barrier Disruption in the Ischemia Stroke Model Via the NF-кB/MMP-9 Signaling Pathway.Mol Neurobiol. 2023 Jul;60(7):3824-3838. doi: 10.1007/s12035-023-03318-7. Epub 2023 Mar 22. Mol Neurobiol. 2023. PMID: 36949221
-
Specific role of tight junction proteins claudin-5, occludin, and ZO-1 of the blood-brain barrier in a focal cerebral ischemic insult.J Mol Neurosci. 2011 Jun;44(2):130-9. doi: 10.1007/s12031-011-9496-4. Epub 2011 Feb 12. J Mol Neurosci. 2011. PMID: 21318404
-
MicroRNA-126-3p/-5p Overexpression Attenuates Blood-Brain Barrier Disruption in a Mouse Model of Middle Cerebral Artery Occlusion.Stroke. 2020 Feb;51(2):619-627. doi: 10.1161/STROKEAHA.119.027531. Epub 2019 Dec 11. Stroke. 2020. PMID: 31822249
-
Ligustrazine Alleviates Blood-Brain Barrier Damage by Downregulating Expression of miR-297c-5p.CNS Neurosci Ther. 2025 May;31(5):e70367. doi: 10.1111/cns.70367. CNS Neurosci Ther. 2025. PMID: 40406932 Free PMC article.
Cited by
-
Small Extracellular Vesicles From Hypoxia-Neuron Maintain Blood-Brain Barrier Integrity.Stroke. 2025 Jun;56(6):1569-1580. doi: 10.1161/STROKEAHA.124.048446. Epub 2025 Apr 2. Stroke. 2025. PMID: 40171669 Free PMC article.
-
Mapping dynamic molecular changes in hippocampal subregions after traumatic brain injury through spatial proteomics.Clin Proteomics. 2024 May 12;21(1):32. doi: 10.1186/s12014-024-09485-6. Clin Proteomics. 2024. PMID: 38735925 Free PMC article.
References
-
- Modarres HP, Janmaleki M, Novin M, et al. In vitro models and systems for evaluating the dynamics of drug delivery to the healthy and diseased brain. J Control Releas. 2018;273:108–30. - PubMed
-
- Li M, Wen Y, Zhang R, et al. Adenoviral vector-induced silencing of RGMa attenuates blood-brain barrier dysfunction in a rat model of MCAO/reperfusion. Brain Res Bull. 2018;142:54–62. - PubMed
-
- Wolburg H, Lippoldt A. Tight junctions of the blood-brain barrier: development, composition and regulation. Vascul Pharmacol. 2002;38:323–37. - PubMed
LinkOut - more resources
Full Text Sources
