Paroxetine Attenuates Chondrocyte Pyroptosis and Inhibits Osteoclast Formation by Inhibiting NF-κB Pathway Activation to Delay Osteoarthritis Progression
- PMID: 37605762
- PMCID: PMC10440089
- DOI: 10.2147/DDDT.S417598
Paroxetine Attenuates Chondrocyte Pyroptosis and Inhibits Osteoclast Formation by Inhibiting NF-κB Pathway Activation to Delay Osteoarthritis Progression
Abstract
Background: Osteoarthritis (OA), a common chronic joint disease, is characterized by cartilage degeneration and subchondral bone reconstruction. NF-κB signaling pathway-activated inflammation and NLRP3-induced pyroptosis play essential roles in the development of OA. In this study, we examine whether paroxetine can inhibit pyroptosis and reduce osteoclast formation, thereby delaying the destruction of knee joints.
Methods: We employed high-density cultures, along with quantitative polymerase chain reactions and Western blotting techniques, to investigate the effects of paroxetine on extracellular matrix synthesis and degradation. The expression levels of NF-κB and pyroptosis-related signaling pathway proteins were examined by Western blotting and immunofluorescence. Furthermore, the impact of paroxetine on RANKL-induced osteoclast formation was evaluated through TRAP staining and F-actin ring fluorescence detection. To investigate the role of paroxetine in vivo, we constructed a mouse model with destabilization of the medial meniscus (DMM) surgery. Safranin O-Fast Green staining, Hematoxylin-Eosin staining, and immunohistochemistry were conducted to observe the extent of knee joint cartilage deformation. In addition, TRAP staining was used to observe the formation of osteoclasts in the subchondral bone.
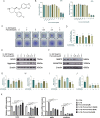
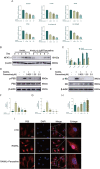
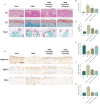
Results: In the in vitro experiments with ATDC5, paroxetine treatment attenuated IL-1β-induced activation of the pyroptosis-related pathway and suppressed extracellular matrix catabolism by inhibiting the NF-kB signaling pathway. In addition, paroxetine treatment decreased the expression of RANKL-induced osteoclast marker genes and reduced osteoclast formation. In animal experiments conducted in vivo, mice treated with paroxetine exhibited thicker knee cartilage with a smoother surface compared to the DMM group. Additionally, the formation of osteoclasts in the subchondral bone was reduced in the paroxetine-treated mice. Further analysis revealed that paroxetine treatment played a role in preserving the balance of the extracellular matrix and delaying knee joint degeneration.
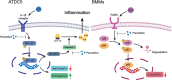
Conclusion: Paroxetine can inhibit pyroptosis and reduce osteoclast formation via inhibiting the NF-κB signaling pathway, suggesting that it may have therapeutic effects in patients with OA.
Keywords: inflammation; osteoarthritis; osteoclasts; paroxetine; pyroptosis.
© 2023 Zheng et al.
Conflict of interest statement
The authors declare no competing interests in this work.
Figures



Similar articles
-
Amentoflavone maintaining extracellular matrix homeostasis and inhibiting subchondral bone loss in osteoarthritis by inhibiting ERK, JNK and NF-κB signaling pathways.J Orthop Surg Res. 2024 Oct 15;19(1):662. doi: 10.1186/s13018-024-05075-2. J Orthop Surg Res. 2024. PMID: 39407273 Free PMC article.
-
Metformin attenuates osteoclast-mediated abnormal subchondral bone remodeling and alleviates osteoarthritis via AMPK/NF-κB/ERK signaling pathway.PLoS One. 2021 Dec 16;16(12):e0261127. doi: 10.1371/journal.pone.0261127. eCollection 2021. PLoS One. 2021. PMID: 34914744 Free PMC article.
-
Morroniside attenuates apoptosis and pyroptosis of chondrocytes and ameliorates osteoarthritic development by inhibiting NF-κB signaling.J Ethnopharmacol. 2021 Feb 10;266:113447. doi: 10.1016/j.jep.2020.113447. Epub 2020 Oct 3. J Ethnopharmacol. 2021. PMID: 33022338
-
Pyroptosis in Osteoarthritis: Molecular Mechanisms and Therapeutic Implications.J Inflamm Res. 2024 Feb 8;17:791-803. doi: 10.2147/JIR.S445573. eCollection 2024. J Inflamm Res. 2024. PMID: 38348279 Free PMC article. Review.
-
Molecular mechanisms and potential applications of chondroitin sulphate in managing post-traumatic osteoarthritis.Reumatologia. 2023;61(5):395-407. doi: 10.5114/reum/172211. Epub 2023 Oct 31. Reumatologia. 2023. PMID: 37970120 Free PMC article. Review.
Cited by
-
The common link between sleep apnea syndrome and osteoarthritis: a literature review.Front Med (Lausanne). 2024 Aug 21;11:1401309. doi: 10.3389/fmed.2024.1401309. eCollection 2024. Front Med (Lausanne). 2024. PMID: 39234045 Free PMC article. Review.
-
Research progress on mesenchymal stem cell‑derived exosomes in the treatment of osteoporosis induced by knee osteoarthritis (Review).Int J Mol Med. 2025 Oct;56(4):160. doi: 10.3892/ijmm.2025.5601. Epub 2025 Aug 1. Int J Mol Med. 2025. PMID: 40747674 Free PMC article. Review.
-
Osteoimmunology in Osteoarthritis: Unraveling the Interplay of Immunity, Inflammation, and Joint Degeneration.J Inflamm Res. 2025 Mar 19;18:4121-4142. doi: 10.2147/JIR.S514002. eCollection 2025. J Inflamm Res. 2025. PMID: 40125089 Free PMC article. Review.
-
Pyroptosis for osteoarthritis treatment: insights into cellular and molecular interactions inflammatory.Front Immunol. 2025 Apr 1;16:1556990. doi: 10.3389/fimmu.2025.1556990. eCollection 2025. Front Immunol. 2025. PMID: 40236711 Free PMC article. Review.
-
Systemic Lupus Erythematosus Exacerbates Hip Arthritis by Promoting Chondrocyte Pyroptosis in the Femoral Head via Activating the NF-κB Pathway.J Cell Mol Med. 2025 Apr;29(7):e70531. doi: 10.1111/jcmm.70531. J Cell Mol Med. 2025. PMID: 40179133 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

