Alveolar type II epithelial cell FASN maintains lipid homeostasis in experimental COPD
- PMID: 37606038
- PMCID: PMC10543729
- DOI: 10.1172/jci.insight.163403
Alveolar type II epithelial cell FASN maintains lipid homeostasis in experimental COPD
Abstract
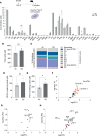
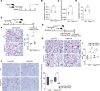
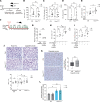
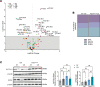
Alveolar epithelial type II (AEC2) cells strictly regulate lipid metabolism to maintain surfactant synthesis. Loss of AEC2 cell function and surfactant production are implicated in the pathogenesis of the smoking-related lung disease chronic obstructive pulmonary disease (COPD). Whether smoking alters lipid synthesis in AEC2 cells and whether altering lipid metabolism in AEC2 cells contributes to COPD development are unclear. In this study, high-throughput lipidomic analysis revealed increased lipid biosynthesis in AEC2 cells isolated from mice chronically exposed to cigarette smoke (CS). Mice with a targeted deletion of the de novo lipogenesis enzyme, fatty acid synthase (FASN), in AEC2 cells (FasniΔAEC2) exposed to CS exhibited higher bronchoalveolar lavage fluid (BALF) neutrophils, higher BALF protein, and more severe airspace enlargement. FasniΔAEC2 mice exposed to CS had lower levels of key surfactant phospholipids but higher levels of BALF ether phospholipids, sphingomyelins, and polyunsaturated fatty acid-containing phospholipids, as well as increased BALF surface tension. FasniΔAEC2 mice exposed to CS also had higher levels of protective ferroptosis markers in the lung. These data suggest that AEC2 cell FASN modulates the response of the lung to smoke by regulating the composition of the surfactant phospholipidome.
Keywords: COPD; Intermediary metabolism; Metabolism; Pulmonary surfactants; Pulmonology.
Conflict of interest statement
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

