Species-specific roles for the MAFA and MAFB transcription factors in regulating islet β cell identity
- PMID: 37606041
- PMCID: PMC10543725
- DOI: 10.1172/jci.insight.166386
Species-specific roles for the MAFA and MAFB transcription factors in regulating islet β cell identity
Abstract
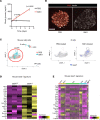
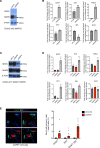
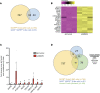
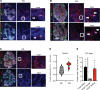
Type 2 diabetes (T2D) is associated with compromised identity of insulin-producing pancreatic islet β cells, characterized by inappropriate production of other islet cell-enriched hormones. Here, we examined how hormone misexpression was influenced by the MAFA and MAFB transcription factors, closely related proteins that maintain islet cell function. Mice specifically lacking MafA in β cells demonstrated broad, population-wide changes in hormone gene expression with an overall gene signature closely resembling islet gastrin+ (Gast+) cells generated under conditions of chronic hyperglycemia and obesity. A human β cell line deficient in MAFB, but not one lacking MAFA, also produced a GAST+ gene expression pattern. In addition, GAST was detected in human T2D β cells with low levels of MAFB. Moreover, evidence is provided that human MAFB can directly repress GAST gene transcription. These results support a potentially novel, species-specific role for MafA and MAFB in maintaining adult mouse and human β cell identity, respectively. Here, we discuss the possibility that induction of Gast/GAST and other non-β cell hormones, by reduction in the levels of these transcription factors, represents a dysfunctional β cell signature.
Keywords: Beta cells; Cell Biology; Diabetes; Endocrinology; Islet cells.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
- UC4 DK104211/DK/NIDDK NIH HHS/United States
- UC4 DK108120/DK/NIDDK NIH HHS/United States
- P30 HD015052/HD/NICHD NIH HHS/United States
- U01 DK120456/DK/NIDDK NIH HHS/United States
- P30 EY008126/EY/NEI NIH HHS/United States
- P30 DK020593/DK/NIDDK NIH HHS/United States
- F32 DK109577/DK/NIDDK NIH HHS/United States
- R24 DK106755/DK/NIDDK NIH HHS/United States
- U24 DK059637/DK/NIDDK NIH HHS/United States
- R01 DK090570/DK/NIDDK NIH HHS/United States
- K01 DK133533/DK/NIDDK NIH HHS/United States
- K08 DK132507/DK/NIDDK NIH HHS/United States
- P30 DK058404/DK/NIDDK NIH HHS/United States
- P30 CA068485/CA/NCI NIH HHS/United States
- R01 DK110276/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

