Circulating miR-146a predicts glucocorticoid response in thyroid eye disease
- PMID: 37606076
- PMCID: PMC10563606
- DOI: 10.1530/ETJ-23-0083
Circulating miR-146a predicts glucocorticoid response in thyroid eye disease
Abstract
Objective: Thyroid eye disease (TED) is an immune-mediated disorder of the eye. Intravenous glucocorticoid (GC) is the first-line treatment for patients with active moderate-to-severe TED. However, the response rate is between 50% and 80%. There are still no simple and reliable markers of responsiveness to GC therapy. We aimed to explore the possible role of miR-146a and miR-21 as predictors of responsiveness to GC treatment in TED.
Methods: We carried out a prospective longitudinal study on 30 consecutive adult patients with active moderate-to-severe TED and eligible for GC therapy. All patients received the standard GC treatment with methylprednisolone i.v. In cases of progressive worsening of Gorman Score for diplopia or with duction restriction <30° in at least two consecutive controls, patients also underwent orbital radiotherapy. Response to GC treatment was defined as a decrease of two or more points in the clinical activity score (CAS) or CAS <4/10 at 24 weeks. Circulating miRNAs were extracted from patients' serum and quantified by real-time PCR.
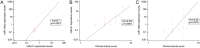
Results: Twenty-three (77%) patients responded to GC. Thyroid surgery, higher CAS, greater proptosis and higher pre-treatment circulating levels of miR-146a emerged as predictive factors of responsiveness to GC. A ROC analysis revealed that miR-146a could predict responsiveness to GC with a positive predictive value of 100%.
Conclusion: This is the first study investigating the role of pre-treatment circulating miR-21 and miR-146a to predict responsiveness to GC in TED. miR-146a emerged as a simple, objective, new marker of GC sensitivity that could be used to avoid ineffective administration of GC therapy to TED patients.
Keywords: glucocorticoid response; glucocorticoid sensitivity; miR-146a; miR-21; microRNA; thyroid eye disease.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Prummel MF, Bakker A, Wiersinga WM, Baldeschi L, Mourits MP, Kendall-Taylor P, Perros P, Neoh C, Dickinson AJ, Lazarus JH, et al.Multi-center study on the characteristics and treatment strategies of patients with Graves' orbitopathy: the first European Group on Graves' orbitopathy experience. European Journal of Endocrinology 2003148491–495. (10.1530/eje.0.1480491) - DOI - PubMed
-
- Bartalena L, Baldeschi L, Dickinson A, Eckstein A, Kendall-Taylor P, Marcocci C, Mourits M, Perros P, Boboridis K, Boschi A, et al.Consensus statement of the European Group on Graves' orbitopathy (EUGOGO) on management of GO. European Journal of Endocrinology 2008158273–285. (10.1530/EJE-07-0666) - DOI - PubMed
-
- Rundle FF & Wilson CW. Development and course of exophthalmos and ophthalmoplegia in Graves' disease with special reference to the effect of thyroidectomy. Clinical Science 19455177–194. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

