Oral Lipid Nanocrystal Amphotericin B for Cryptococcal Meningitis: A Randomized Clinical Trial
- PMID: 37606364
- PMCID: PMC10724459
- DOI: 10.1093/cid/ciad440
Oral Lipid Nanocrystal Amphotericin B for Cryptococcal Meningitis: A Randomized Clinical Trial
Abstract
Background: Amphotericin B is the gold standard treatment for severe mycoses. A new orally delivered, less-toxic formulation of amphotericin has been developed.
Methods: In our randomized clinical trial, we tested oral lipid nanocrystal (LNC) amphotericin B (MAT2203, Matinas Biopharma) vs intravenous (IV) amphotericin for human immunodeficiency virus-associated cryptococcal meningitis in 4 sequential cohorts. Two pilot cohorts assessed safety and tolerability (n = 10 each), and 2 cohorts assessed efficacy with/without 2 IV loading doses (n = 40 each). The experimental arm received 1.8 g/d oral LNC amphotericin through 2 weeks with 100 mg/kg/d flucytosine, then 1.2 g/d LNC amphotericin through 6 weeks. The randomized control arm (n = 41) received 7 days of IV amphotericin with flucytosine, then 7 days of fluconazole 1200 mg/d. The primary end point was cerebrospinal fluid (CSF) early fungicidal activity (EFA).
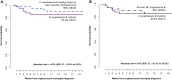
Results: We randomized 80 participants to oral LNC amphotericin + flucytosine with (n = 40) and without (n = 40) 2 IV loading doses and 41 control participants to IV amphotericin + flucytosine. Mean EFA was 0.40 log10 colony-forming units (CFU)/mL/d for all-oral LNC amphotericin, 0.42 log10 Cryptococcus CFU/mL/d for oral LNC amphotericin with IV loading doses, and 0.46 log10 CFU/mL/d for IV amphotericin controls. LNC amphotericin groups achieved 2-week CSF sterility in 63% (44 of 70) vs 68% (23 of 34) of controls. The 18-week survival was 85% (34 of 40) with all-oral LNC amphotericin, 90% (36 of 40) with oral LNC amphotericin given IV loading doses, and 85% (35 of 41) with IV amphotericin.Grade 3-4 laboratory adverse events occurred less frequently in LNC amphotericin groups (41%) than the IV amphotericin group (61%, P = .05), particularly for anemia (21% vs 44%; P = .01) and potassium (5% vs 17%; P = .04).
Conclusions: This new oral amphotericin B LNC formulation appears promising for cryptococcal meningitis with antifungal activity, similar survival, and less toxicity than IV amphotericin.
Clinical trials registration: NCT04031833.
Keywords: AIDS-related opportunistic infection; HIV; amphotericin B; cryptococcal meningitis; randomized controlled trial.
Published by Oxford University Press on behalf of Infectious Diseases Society of America 2023.
Conflict of interest statement
Potential conflicts of interest. T. M. is an employee of Matinas Biopharma and has an equity interest and receives support for attending meetings and/or travel and reports a role as board director for Apilli Therapeutics and GoodCap Pharmaceuticals. P. R. W. reports grants or contracts from Matinas BioPharma. D. R. B. serves on the scientific advisory board for Sfunga Therapeutics, who is developing a different antifungal therapeutic. All remaining authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures



Similar articles
-
Combination flucytosine and high-dose fluconazole compared with fluconazole monotherapy for the treatment of cryptococcal meningitis: a randomized trial in Malawi.Clin Infect Dis. 2010 Feb 1;50(3):338-44. doi: 10.1086/649861. Clin Infect Dis. 2010. PMID: 20038244 Free PMC article. Clinical Trial.
-
Single-Dose Liposomal Amphotericin B Treatment for Cryptococcal Meningitis.N Engl J Med. 2022 Mar 24;386(12):1109-1120. doi: 10.1056/NEJMoa2111904. N Engl J Med. 2022. PMID: 35320642 Free PMC article. Clinical Trial.
-
Combination antifungal therapy for cryptococcal meningitis.N Engl J Med. 2013 Apr 4;368(14):1291-1302. doi: 10.1056/NEJMoa1110404. N Engl J Med. 2013. PMID: 23550668 Free PMC article. Clinical Trial.
-
An updated systematic review of HIV-associated cryptococcal meningitis treatment strategies.HIV Med. 2023 Apr;24(4):507-512. doi: 10.1111/hiv.13412. Epub 2022 Sep 19. HIV Med. 2023. PMID: 36123803 Free PMC article.
-
Cryptococcal meningitis in patients with the acquired immunodeficiency syndrome.Pharmacotherapy. 1996 Jul-Aug;16(4):547-61. Pharmacotherapy. 1996. PMID: 8840361 Review.
Cited by
-
Efficacy of an oral lipid nanocrystal formulation of amphotericin B (MAT2203) in the neutropenic mouse model of pulmonary mucormycosis.Antimicrob Agents Chemother. 2024 Jun 5;68(6):e0154023. doi: 10.1128/aac.01540-23. Epub 2024 Apr 30. Antimicrob Agents Chemother. 2024. PMID: 38687015 Free PMC article.
-
Comparison of Early Fungicidal Activity and Mortality Between Daily Liposomal Amphotericin B and Daily Amphotericin B Deoxycholate for Cryptococcal Meningitis.Clin Infect Dis. 2025 Feb 5;80(1):153-159. doi: 10.1093/cid/ciae326. Clin Infect Dis. 2025. PMID: 38943665 Free PMC article.
-
Clinical Mycology Today: Emerging Challenges and Opportunities.Open Forum Infect Dis. 2024 Jun 27;11(7):ofae363. doi: 10.1093/ofid/ofae363. eCollection 2024 Jul. Open Forum Infect Dis. 2024. PMID: 39045011 Free PMC article. Review.
-
Experience of research nurses with oral encochleated amphotericin B for treatment of cryptococcal meningitis in a resource-limited setting.BMC Infect Dis. 2025 Jul 15;25(1):920. doi: 10.1186/s12879-025-11319-1. BMC Infect Dis. 2025. PMID: 40665233 Free PMC article.
-
Nanotechnology in the Diagnosis and Treatment of Antibiotic-Resistant Infections.Antibiotics (Basel). 2024 Jan 25;13(2):121. doi: 10.3390/antibiotics13020121. Antibiotics (Basel). 2024. PMID: 38391507 Free PMC article. Review.
References
-
- World Health Organization . Guidelines for diagnosing, preventing and managing cryptococcal disease among adults, adolescents and children living with HIV. Available at: https://www.who.int/publications/i/item/9789240052178. Accessed 27 June 2022. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

