Exploring Parametric and Mechanistic Differences between Expi293FTM and ExpiCHO-STM Cells for Transient Antibody Production Optimization
- PMID: 37606437
- PMCID: PMC10443273
- DOI: 10.3390/antib12030053
Exploring Parametric and Mechanistic Differences between Expi293FTM and ExpiCHO-STM Cells for Transient Antibody Production Optimization
Abstract
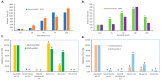
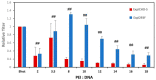
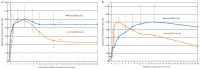
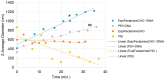
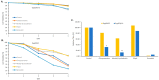
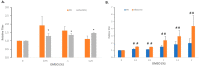
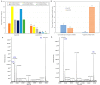
Rapidly producing drug-like antibody therapeutics for lead molecule discovery and candidate optimization is typically accomplished by large-scale transient gene expression technologies (TGE) with cultivated mammalian cells. The TGE methodologies have been extensively developed over the past three decades, yet produce significantly lower yields than the stable cell line approach, facing the technical challenge of achieving universal high expression titers for a broad range of antibodies and therapeutics modalities. In this study, we explored various parameters for antibody production in the TGE cell host Expi293FTM and ExpiCHO-STM with the transfection reagents ExpiFectamineTM and polyethylenimine. We discovered that there are significant differences between Expi293FTM and ExpiCHO-STM cells with regards to DNA complex formation time and ratio, complex formation buffers, DNA complex uptake trafficking routes, responses to dimethyl sulfoxide and cell cycle inhibitors, as well as light-chain isotype expression preferences. This investigation mechanistically dissected the TGE processes and provided a new direction for future transient antibody production optimization.
Keywords: CHO cells; DNA uptake trafficking pathways; HEK cells; Kappa and lambda light chain; antibody production; transfection reagents; transient gene expression.
Conflict of interest statement
All authors are employed by Pfizer research. All authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
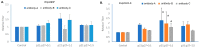
Similar articles
-
Comparison of different transient gene expression systems for the production of a new humanized anti-HER2 monoclonal antibody (Hersintuzumab).Daru. 2023 Dec;31(2):221-231. doi: 10.1007/s40199-023-00477-9. Epub 2023 Sep 11. Daru. 2023. PMID: 37695454 Free PMC article.
-
Transient CHO expression platform for robust antibody production and its enhanced N-glycan sialylation on therapeutic glycoproteins.Biotechnol Prog. 2019 Jan;35(1):e2724. doi: 10.1002/btpr.2724. Epub 2018 Oct 27. Biotechnol Prog. 2019. PMID: 30299005
-
A high cell density transient transfection system for therapeutic protein expression based on a CHO GS-knockout cell line: process development and product quality assessment.Biotechnol Bioeng. 2015 May;112(5):977-86. doi: 10.1002/bit.25514. Epub 2015 Mar 16. Biotechnol Bioeng. 2015. PMID: 25502369
-
Advancements in mammalian cell transient gene expression (TGE) technology for accelerated production of biologics.Crit Rev Biotechnol. 2018 Sep;38(6):918-940. doi: 10.1080/07388551.2017.1419459. Epub 2018 Jan 2. Crit Rev Biotechnol. 2018. PMID: 29295632 Review.
-
Recombinant protein production by large-scale transient gene expression in mammalian cells: state of the art and future perspectives.Biotechnol Lett. 2007 May;29(5):677-84. doi: 10.1007/s10529-006-9297-y. Epub 2007 Jan 19. Biotechnol Lett. 2007. PMID: 17235486 Review.
Cited by
-
Impact of Light-Chain Variants on the Expression of Therapeutic Monoclonal Antibodies in HEK293 and CHO Cells.Antibodies (Basel). 2025 Jun 24;14(3):53. doi: 10.3390/antib14030053. Antibodies (Basel). 2025. PMID: 40700293 Free PMC article.
-
CD45 sequestration lowers the signaling threshold in lymphocytes and enhances anti-tumor immunity.bioRxiv [Preprint]. 2025 Aug 1:2025.07.29.667400. doi: 10.1101/2025.07.29.667400. bioRxiv. 2025. PMID: 40766423 Free PMC article. Preprint.
-
Tyrosine Sulfation at Antibody Light Chain CDR-1 Increases Binding Affinity and Neutralization Potency to Interleukine-4.Int J Mol Sci. 2024 Feb 5;25(3):1931. doi: 10.3390/ijms25031931. Int J Mol Sci. 2024. PMID: 38339208 Free PMC article.
-
Metabolic Engineering of Glycofusion Bispecific Antibodies for α-Dystroglycanopathies.Antibodies (Basel). 2024 Oct 7;13(4):83. doi: 10.3390/antib13040083. Antibodies (Basel). 2024. PMID: 39449325 Free PMC article.
References
-
- Baldi L., Hacker D.L., Adam M., Wurm F.M. Recombinant protein production by large-scale transient gene expression in mammalian cells: State of the art and future perspectives. Biotechnol. Lett. 2007;29:677–684. - PubMed
-
- Geisse S., Voedisch B. Transient expression technologies: Past, present, and future. Methods Mol. Biol. 2012;899:203–219. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

