Tremor Induced by Cyclosporine, Tacrolimus, Sirolimus, or Everolimus: A Review of the Literature
- PMID: 37606750
- PMCID: PMC10676343
- DOI: 10.1007/s40268-023-00428-4
Tremor Induced by Cyclosporine, Tacrolimus, Sirolimus, or Everolimus: A Review of the Literature
Abstract
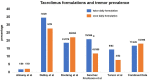
Calcineurin inhibitors such as cyclosporine and tacrolimus are immunosuppressant drugs that are known to induce tremors. Non-calcineurin inhibitors such as sirolimus and everolimus have also reportedly been accompanied by tremors, albeit less likely. However, the prevalence rates reported in the literature are notably wide, and the risk profiles for these drug-induced tremors are less understood. We searched PubMed to extract data on the risk of tremors with these drugs when prescribed for various transplant and non-transplant indications. We ascertained whether the risk of drug-induced tremor is influenced by the underlying diagnosis, dosing formulations, drug concentrations, and blood monitoring. We extracted data on treatment strategies and outcomes for tremors. Articles were primarily screened based on English language publications, abstracts, and studies with n ≥ 5, which included case series, retrospective studies, case-controlled studies, and prospective studies. We found 81 eligible studies comprising 33 cyclosporine, 43 tacrolimus, 6 sirolimus, and 1 everolimus that discussed tremor as an adverse event. In the pooled analysis of studies with n > 100, the incidence of tremor was 17% with cyclosporine, 21.5% with tacrolimus, and 7.8% with sirolimus and everolimus together. Regarding the underlying diagnosis, tremor was more frequently reported in kidney transplant (cyclosporine 28%, tacrolimus 30.1%) and bone marrow transplant (cyclosporine 40%, tacrolimus 41.9%) patients compared with liver transplant (cyclosporine 9%, tacrolimus 11.5%) and nontransplant indications (cyclosporine 21.5%, tacrolimus 11.3%). Most studies did not report whether the risk of tremors correlated with drug concentrations in the blood. The prevalence of tremors when using the twice-daily formulation of tacrolimus was nearly the same as the once-daily formulation (17% vs 18%). Data on individual-level risk factors for tremors were lacking. Except for three studies that found some benefit to maintaining magnesium levels, there were minimal data on treatments and outcomes. A large body of data supports a substantive and wide prevalence of tremor resulting from tacrolimus use followed by cyclosporine, especially in patients receiving a kidney transplant. However, there is little reporting on the patient-related risk factors for tremor, risk relationship with drug concentrations, treatment strategies, and outcomes.
© 2023. The Author(s).
Conflict of interest statement
Aparna Wagle Shukla, Caroline Lunny, Omar Mahboob, Uzair Khalid, Malea Joyce, Nivedita Jha, Nandakumar Nagaraja and Ashutosh M. Shukla have no conflicts of interest that are directly relevant to the content of this article.
Figures


References
-
- Shrestha BM. Two decades of tacrolimus in renal transplant: basic science and clinical evidences. Exp Clin Transplant. 2017;15:1–9. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

