OCE-205, A Novel, Selective Vasopressin Receptor Mixed Agonist-Antagonist: Safety, Tolerability, and Pharmacokinetics/Pharmacodynamics from a Phase 1 Study in Healthy Volunteers
- PMID: 37606870
- PMCID: PMC10514109
- DOI: 10.1007/s40261-023-01299-y
OCE-205, A Novel, Selective Vasopressin Receptor Mixed Agonist-Antagonist: Safety, Tolerability, and Pharmacokinetics/Pharmacodynamics from a Phase 1 Study in Healthy Volunteers
Abstract
Background: OCE-205, a novel, selective vasopressin V1a receptor mixed agonist/antagonist with no V2 receptor activity, may treat the portal hypertension-related complications of end-stage liver disease with an improved therapeutic profile over currently utilized nonselective full-agonist vasopressin analogs.
Objectives: This Phase 1, double-blind, placebo-controlled, within-dose-group randomized trial investigated the safety, tolerability, and pharmacokinetic/pharmacodynamic profiles of OCE-205 in healthy adults.
Methods: Subjects received a single intravenous dose of OCE-205 0.1, 0.3, 0.45, 0.6, or 0.9 mg, or placebo infused over 6 h. Safety and tolerability were assessed, and blood samples were obtained for pharmacokinetic analyses. Sixty-four subjects were randomized and treated.
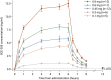
Results: Area under the concentration-time curve (AUC) and maximum plasma concentrations (Cmax) were approximately dose-proportional across doses from 0.1 to 0.9 mg. OCE-205 terminal half-life was ~ 1.5 h. Diastolic, and to a lesser extent systolic, blood pressure increased in all OCE-205 dose groups; pulse rate decreased. Overall changes in mean arterial pressure were similar to changes in diastolic blood pressure. Absolute changes in cardiac output, by echocardiogram, were somewhat dose-dependent, with mean reductions of 3-12% after the 0.9 mg dose, and individual reductions ≤ 20 to 25% across all doses. The most frequent adverse events were abdominal pain, abnormal gastrointestinal sounds, and diarrhea, with no reported cases of mesenteric ischemia. Adverse events were generally mild or moderate in severity.
Conclusion: OCE-205 was safe and well tolerated, with a pharmacodynamic profile achieving submaximal partial agonism consistent with mixed agonism-antagonism of the V1a receptor. OCE-205 shows promise as a treatment for some complications of end-stage liver disease.
© 2023. The Author(s).
Conflict of interest statement
Stan Bukofzer and Geoff Harris are founders of Ocelot Bio, Inc; Stan Bukofzer, Geoff Harris, and William R. Ravis are consultants/advisors to Ocelot Bio, Inc. Yu Bagger was an employee of Ferring Pharmaceuticals A/S at the time of the study.
Figures

References
-
- Biggins SW, Angeli P, Garcia-Tsao G, et al. Diagnosis, evaluation, and management of ascites, spontaneous bacterial peritonitis and hepatorenal syndrome: 2021 practice guidance by the American Association for the Study of Liver Diseases. Hepatology. 2021;74(2):1014–1048. doi: 10.1002/hep.31884. - DOI - PubMed
-
- Harper D, Chandler B. Splanchnic circulation. BJA Educ. 2016;16:66–71. doi: 10.1093/bjaceaccp/mkv017. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

