Recovery of Forearm and Fine Digit Function After Chronic Spinal Cord Injury by Simultaneous Blockade of Inhibitory Matrix Chondroitin Sulfate Proteoglycan Production and the Receptor PTPσ
- PMID: 37606910
- PMCID: PMC10698859
- DOI: 10.1089/neu.2023.0117
Recovery of Forearm and Fine Digit Function After Chronic Spinal Cord Injury by Simultaneous Blockade of Inhibitory Matrix Chondroitin Sulfate Proteoglycan Production and the Receptor PTPσ
Abstract
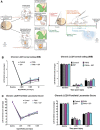
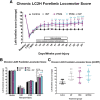
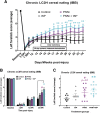
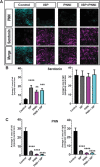
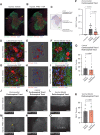
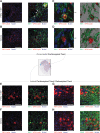
Spinal cord injuries (SCI), for which there are limited effective treatments, result in enduring paralysis and hypoesthesia, in part because of the inhibitory microenvironment that develops and limits regeneration/sprouting, especially during chronic stages. Recently, we discovered that targeted enzymatic removal of the inhibitory chondroitin sulfate proteoglycan (CSPG) component of the extracellular and perineuronal net (PNN) matrix via Chondroitinase ABC (ChABC) rapidly restored robust respiratory function to the previously paralyzed hemi-diaphragm after remarkably long times post-injury (up to 1.5 years) following a cervical level 2 lateral hemi-transection. Importantly, ChABC treatment at cervical level 4 in this chronic model also elicited improvements in gross upper arm function. In the present study, we focused on arm and hand function, seeking to highlight and optimize crude as well as fine motor control of the forearm and digits at lengthy chronic stages post-injury. However, instead of using ChABC, we utilized a novel and more clinically relevant systemic combinatorial treatment strategy designed to simultaneously reduce and overcome inhibitory CSPGs. Following a 3-month upper cervical spinal hemi-lesion using adult female Sprague Dawley rats, we show that the combined treatment had a profound effect on functional recovery of the chronically paralyzed forelimb and paw, as well as on precision movements of the digits. The regenerative and immune system related events that we describe deepen our basic understanding of the crucial role of CSPG-mediated inhibition via the PTPσ receptor in constraining functional synaptic plasticity at lengthy time points following SCI, hopefully leading to clinically relevant translational benefits.
Keywords: CSPGs; axon regeneration; axon sprouting; perineuronal net; receptor PTPσ; spinal cord injury.
Conflict of interest statement
No competing financial interests exist.
Figures



Similar articles
-
Perturbing chondroitin sulfate proteoglycan signaling through LAR and PTPσ receptors promotes a beneficial inflammatory response following spinal cord injury.J Neuroinflammation. 2018 Mar 20;15(1):90. doi: 10.1186/s12974-018-1128-2. J Neuroinflammation. 2018. PMID: 29558941 Free PMC article.
-
Suppressing CSPG/LAR/PTPσ Axis Facilitates Neuronal Replacement and Synaptogenesis by Human Neural Precursor Grafts and Improves Recovery after Spinal Cord Injury.J Neurosci. 2022 Apr 13;42(15):3096-3121. doi: 10.1523/JNEUROSCI.2177-21.2022. Epub 2022 Mar 7. J Neurosci. 2022. PMID: 35256527 Free PMC article.
-
Chondroitin sulfate proteoglycans inhibit oligodendrocyte myelination through PTPσ.Exp Neurol. 2013 Sep;247:113-21. doi: 10.1016/j.expneurol.2013.04.003. Epub 2013 Apr 12. Exp Neurol. 2013. PMID: 23588220
-
Regulation of autophagy by inhibitory CSPG interactions with receptor PTPσ and its impact on plasticity and regeneration after spinal cord injury.Exp Neurol. 2020 Jun;328:113276. doi: 10.1016/j.expneurol.2020.113276. Epub 2020 Mar 4. Exp Neurol. 2020. PMID: 32145250 Free PMC article. Review.
-
Scar-mediated inhibition and CSPG receptors in the CNS.Exp Neurol. 2012 Oct;237(2):370-8. doi: 10.1016/j.expneurol.2012.07.009. Epub 2012 Jul 24. Exp Neurol. 2012. PMID: 22836147 Free PMC article. Review.
Cited by
-
Perineuronal Net Microscopy: From Brain Pathology to Artificial Intelligence.Int J Mol Sci. 2024 Apr 11;25(8):4227. doi: 10.3390/ijms25084227. Int J Mol Sci. 2024. PMID: 38673819 Free PMC article. Review.
-
PTEN inhibition promotes robust growth of bulbospinal respiratory axons and partial recovery of diaphragm function in a chronic model of cervical contusion spinal cord injury.Exp Neurol. 2024 Aug;378:114816. doi: 10.1016/j.expneurol.2024.114816. Epub 2024 May 22. Exp Neurol. 2024. PMID: 38789023 Free PMC article.
-
Pharmacological intervention for chronic phase of spinal cord injury.Neural Regen Res. 2025 May 1;20(5):1377-1389. doi: 10.4103/NRR.NRR-D-24-00176. Epub 2024 Jun 26. Neural Regen Res. 2025. PMID: 38934397 Free PMC article.
-
Spinal cord injury repair based on drug and cell delivery: From remodeling microenvironment to relay connection formation.Mater Today Bio. 2025 Feb 4;31:101556. doi: 10.1016/j.mtbio.2025.101556. eCollection 2025 Apr. Mater Today Bio. 2025. PMID: 40026622 Free PMC article. Review.
-
Schwann Cell-Derived Exosomes Induced Axon Growth after Spinal Cord Injury by Decreasing PTP-σ Activation on CSPGs via the Rho/ROCK Pathway.Neurochem Res. 2024 Aug;49(8):2120-2130. doi: 10.1007/s11064-024-04166-0. Epub 2024 May 31. Neurochem Res. 2024. PMID: 38819695
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
