Effect of Vitamin D Supplements on Relapse or Death in a p53-Immunoreactive Subgroup With Digestive Tract Cancer: Post Hoc Analysis of the AMATERASU Randomized Clinical Trial
- PMID: 37606927
- PMCID: PMC10445201
- DOI: 10.1001/jamanetworkopen.2023.28886
Effect of Vitamin D Supplements on Relapse or Death in a p53-Immunoreactive Subgroup With Digestive Tract Cancer: Post Hoc Analysis of the AMATERASU Randomized Clinical Trial
Abstract
Importance: Recent meta-analyses of randomized clinical trials found that daily vitamin D3 supplementation had beneficial effects on cancer mortality, although the results are still controversial.
Objective: To examine whether vitamin D supplementation reduces the risk of relapse or death in a supgroup of patients with digestive tract cancer who were p53 immunoreactive.
Design, setting, and participants: This was a post hoc subgroup analysis of the AMATERASU randomized, double-blind, placebo-controlled clinical trial. This trial included patients at a single university hospital in Japan with digestive tract cancers between January 2010 and February 2018 followed up for a median (IQR) of 3.5 (2.5-5.3) years to compare the effects of vitamin D supplementation with placebo and was reported in 2019. Patients from among 417 participants in the AMATERASU trial whose residual serum samples were available were included. Data were analyzed from October 20 to November 24, 2022.
Interventions: Vitamin D3 (2000 IU/d) supplementation or placebo.
Main outcomes and measures: The primary outcome was 5-year relapse or death. The subgroup of patients who were p53 immunoreactive was defined by positivity for anti-p53 antibodies in serum and nuclear accumulation of p53 oncosuppressor protein in more than 99% of cancer cells, which is considered a biomarker for p53 missense mutations. Anti-p53 antibody levels were measured using chemiluminescent enzyme immune assay. Immunohistochemical staining data of p53 protein in cancer tissue in pathologic specimens were obtained from a previous study and divided into 4 grades.
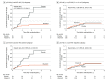
Results: Among 392 patients with digestive tract cancer (mean [SD] age, 66 [10.7] years; 260 males [66.3%]), there were 37 patients with esophageal cancer (9.4%), 170 patients with gastric cancer (43.4%), 2 patients with small bowel cancer (0.5%), and 183 patients with colorectal cancer (46.7%). Serum anti-p53 antibody was detectable in 142 patients (36.2%), and p53-immunohistochemistry grade showed a positive association with serum anti-p53 antibody levels (coefficient = 0.19; P < .001). In the p53-immunoreactive subgroup (80 patients), relapse or death occurred in 9 of 54 patients (16.7%) in the vitamin D group and 14 of 26 patients (53.8%) in the placebo group; 5-year relapse-free survival (RFS) was significantly higher in the vitamin D group (13 patients [80.9%]) than the placebo group (1 patient [30.6%]; hazard ratio [HR], 0.27; 95% CI, 0.11-0.61; P = .002). This was significantly different from 272 patients in the non-p53 immunoreactive subgroup, in which vitamin D had no effect on 5-year RFS (vitamin D: 35 of 158 patients [22.2%] vs placebo: 24 of 114 patients [21.1%]; HR, 1.09; 95% CI, 0.65-1.84) (P for interaction = .005).
Conclusions and relevance: This study found that vitamin D supplementation reduced the risk of relapse or death in the subgroup of patients with digestive tract cancer who were p53 immunoreactive.
Trial registration: Identifier: UMIN000001977.
Conflict of interest statement
Figures



Comment in
-
The Death D-Fying Vitamin D3 for Digestive Tract Cancers-The p53 Antibody Connection.JAMA Netw Open. 2023 Aug 1;6(8):e2328883. doi: 10.1001/jamanetworkopen.2023.28883. JAMA Netw Open. 2023. PMID: 37606930 No abstract available.
Similar articles
-
Effect of Vitamin D Supplementation on Relapse-Free Survival Among Patients With Digestive Tract Cancers: The AMATERASU Randomized Clinical Trial.JAMA. 2019 Apr 9;321(14):1361-1369. doi: 10.1001/jama.2019.2210. JAMA. 2019. PMID: 30964526 Free PMC article. Clinical Trial.
-
Effect of Vitamin D on Relapse-Free Survival in a Subgroup of Patients with p53 Protein-Positive Digestive Tract Cancer: A Post Hoc Analysis of the AMATERASU Trial.Cancer Epidemiol Biomarkers Prev. 2020 Feb;29(2):406-413. doi: 10.1158/1055-9965.EPI-19-0986. Epub 2019 Dec 23. Cancer Epidemiol Biomarkers Prev. 2020. PMID: 31871108 Clinical Trial.
-
Vitamin D Supplementation Regulates Postoperative Serum Levels of PD-L1 in Patients with Digestive Tract Cancer and Improves Survivals in the Highest Quintile of PD-L1: A Post Hoc Analysis of the AMATERASU Randomized Controlled Trial.Nutrients. 2021 Jun 9;13(6):1987. doi: 10.3390/nu13061987. Nutrients. 2021. PMID: 34207794 Free PMC article. Clinical Trial.
-
Vitamin D supplementation to prevent acute respiratory infections: a systematic review and meta-analysis of aggregate data from randomised controlled trials.Lancet Diabetes Endocrinol. 2021 May;9(5):276-292. doi: 10.1016/S2213-8587(21)00051-6. Epub 2021 Mar 30. Lancet Diabetes Endocrinol. 2021. PMID: 33798465
-
Vitamin D supplementation for sickle cell disease.Cochrane Database Syst Rev. 2020 May 28;5(5):CD010858. doi: 10.1002/14651858.CD010858.pub3. Cochrane Database Syst Rev. 2020. PMID: 32462740 Free PMC article.
Cited by
-
Nutritional Management of Oncological Symptoms: A Comprehensive Review.Nutrients. 2023 Dec 11;15(24):5068. doi: 10.3390/nu15245068. Nutrients. 2023. PMID: 38140327 Free PMC article. Review.
-
Long Follow-Up Times Weaken Observational Diet-Cancer Study Outcomes: Evidence from Studies of Meat and Cancer Risk.Nutrients. 2023 Dec 21;16(1):26. doi: 10.3390/nu16010026. Nutrients. 2023. PMID: 38201857 Free PMC article.
-
A Narrative Review: Repurposing Metformin as a Potential Therapeutic Agent for Oral Cancer.Cancers (Basel). 2024 Aug 29;16(17):3017. doi: 10.3390/cancers16173017. Cancers (Basel). 2024. PMID: 39272875 Free PMC article. Review.
-
Vitamin D: Evidence-Based Health Benefits and Recommendations for Population Guidelines.Nutrients. 2025 Jan 14;17(2):277. doi: 10.3390/nu17020277. Nutrients. 2025. PMID: 39861407 Free PMC article. Review.
-
Vitamin D, Gut Microbiota, and Cancer Immunotherapy-A Potentially Effective Crosstalk.Int J Mol Sci. 2025 Jul 22;26(15):7052. doi: 10.3390/ijms26157052. Int J Mol Sci. 2025. PMID: 40806182 Free PMC article. Review.
References
-
- International Agency for Research on Cancer . Estimated number of new cases from 2020. to 2040, both sexes, age [0-85+]. Accessed January 7, 2023. https://gco.iarc.fr/tomorrow/en/dataviz/isotype
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

