Intravenous Cefazolin Achieves Sustained High Interstitial Concentrations in Open Lower Extremity Fractures
- PMID: 37606954
- PMCID: PMC10776155
- DOI: 10.1097/CORR.0000000000002808
Intravenous Cefazolin Achieves Sustained High Interstitial Concentrations in Open Lower Extremity Fractures
Abstract
Background: Infection remains a serious clinical concern in patients with open fractures, despite timely antibiotic administration and surgical debridement. Soft tissue and periosteal stripping may alter local tissue homeostasis and antibiotic pharmacokinetics in the injured limb. The tissue (interstitial) concentration of intravenously administered antibiotics at an open fracture site has not been characterized using direct sampling techniques.
Question/purpose: We performed this study to evaluate the concentration and pharmacokinetics of intravenously delivered cefazolin at an open fracture site after surgical debridement.
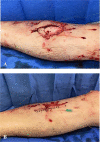
Methods: Twelve patients with an open fracture distal to the knee who presented at a regional Level I trauma center were approached for enrollment in this nonrandomized, observational study. Of the 12 patients, eight adults (one female, seven male) with a median age of 32 years (range 23 to 51 years) were enrolled and underwent successful sample collection for analysis. Three patients had incomplete datasets because of equipment malfunction and one elected not to participate. Seven patients had open tibia fractures, and one patient had an open fibula fracture associated with a closed tibia fracture. There were six Gustilo-Anderson Type II injuries and two Type IIIA injuries. Empiric antibiotics were administered in the prehospital setting or in the emergency department according to institutional protocol. When patients were taken to the operating room, a 2-g intravenous dose of cefazolin was administered. After surgical debridement, fracture stabilization, and wound closure, a microdialysis catheter was placed transdermally into the injury zone (within 5 cm of the fracture site) and a second catheter was placed in the contralateral uninjured (control) limb. Additional doses of cefazolin were administered every 8 hours postoperatively. Baseline and periodic interstitial fluid and whole blood (plasma) samples were collected in the operating room and at prespecified times for 24 hours postoperatively. Free cefazolin in the interstitial fluid and plasma samples were analyzed by ultra-high-performance liquid chromatography using C 18 column separation with quadrupole time-of-flight mass spectrometry detection. Data from the second postoperative dose of cefazolin were used to characterize pharmacokinetic parameters through a noncompartmental analysis using time-concentration curves of free cefazolin and assuming first-order elimination. For pharmacodynamic analyses, the modal cefazolin minimum inhibitory concentration (MIC) of Staphylococcus aureus (1 µg/mL) was used.
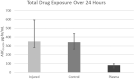
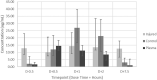
Results: With the samples available, no difference was observed in the median free cefazolin exposure over 24 hours ( f area under the curve [AUC] 0→24hrs ) between injured limbs (352 μg∙hr/mL [IQR 284 to 594 μg∙hr/mL]) and uninjured limbs (341 μg∙hr/mL [IQR 263 to 438 μg∙hr/mL]; p = 0.64). The median time to achieve the maximum concentration of free cefazolin ( f T max ) for injured limbs was delayed (2.7 hours [IQR 2.2 to 3.1 hours]) compared with control limbs (1.7 hours [IQR 1.2 to 2.0 hours]; p = 0.046). The time to the maximum concentration for plasma was not different from that of control limbs (p = 0.08). The time the cefazolin concentration was above the modal S. aureus MIC (T > MIC) in the injured and control limbs over 24 hours was 100% (IQR 100% to 100%) and 100% (IQR 97% to 100%), respectively.
Conclusion: These preliminary findings suggest that current prophylactic cefazolin dosing regimens result in successful antibiotic delivery to the traumatized limb in moderately severe open fractures. Although cefazolin delivery to open-fracture wound beds was delayed compared with healthy tissues, the cefazolin concentration was sustained above the European Union Committee Antimicrobial Susceptibility Testing modal MIC for S. aureus , demonstrating a high likelihood of a prophylactic antimicrobial environment at an open fracture site with this empiric antimicrobial regimen. Importantly, patients in this analysis had Gustilo-Anderson Types II and IIIA injuries. Further research with a larger patient cohort is needed to determine whether antibiotic delivery to traumatized soft tissues in patients with higher-grade open fractures (Gustilo-Anderson Types IIIB and IIIC) demonstrates similar pharmacokinetic characteristics.
Level of evidence: Level II, therapeutic study.
Copyright © 2023 by the Association of Bone and Joint Surgeons.
Conflict of interest statement
Each author certifies that there are no funding or commercial associations (consultancies, stock ownership, equity interest, patent/licensing arrangements, etc.) that might pose a conflict of interest in connection with the submitted article related to the author or any immediate family members. All ICMJE Conflict of Interest Forms for authors and Clinical Orthopaedics and Related Research ® editors and board members are on file with the publication and can be viewed on request.
Figures
Comment in
-
CORR Insights®: Intravenous Cefazolin Achieves Sustained High Interstitial Concentrations in Open Lower Extremity Fractures.Clin Orthop Relat Res. 2024 Feb 1;482(2):384-385. doi: 10.1097/CORR.0000000000002856. Epub 2023 Sep 7. Clin Orthop Relat Res. 2024. PMID: 37678398 Free PMC article. No abstract available.
Similar articles
-
Does Augmenting Irradiated Autografts With Free Vascularized Fibula Graft in Patients With Bone Loss From a Malignant Tumor Achieve Union, Function, and Complication Rate Comparably to Patients Without Bone Loss and Augmentation When Reconstructing Intercalary Resections in the Lower Extremity?Clin Orthop Relat Res. 2025 Jun 26. doi: 10.1097/CORR.0000000000003599. Online ahead of print. Clin Orthop Relat Res. 2025. PMID: 40569278
-
Timing of antibiotic administration, wound debridement, and the stages of reconstructive surgery for open long bone fractures of the upper and lower limbs.Cochrane Database Syst Rev. 2022 Apr 1;4(4):CD013555. doi: 10.1002/14651858.CD013555.pub2. Cochrane Database Syst Rev. 2022. PMID: 35363374 Free PMC article.
-
The effect of sample site and collection procedure on identification of SARS-CoV-2 infection.Cochrane Database Syst Rev. 2024 Dec 16;12(12):CD014780. doi: 10.1002/14651858.CD014780. Cochrane Database Syst Rev. 2024. PMID: 39679851 Free PMC article.
-
What Are the Complications, Function, and Survival of Tumor-devitalized Autografts Used in Patients With Limb-sparing Surgery for Bone and Soft Tissue Tumors? A Japanese Musculoskeletal Oncology Group Multi-institutional Study.Clin Orthop Relat Res. 2023 Nov 1;481(11):2110-2124. doi: 10.1097/CORR.0000000000002720. Epub 2023 Jun 14. Clin Orthop Relat Res. 2023. PMID: 37314384 Free PMC article.
-
Negative pressure wound therapy in grade IIIB tibial fractures: fewer infections and fewer flap procedures?Clin Orthop Relat Res. 2015 May;473(5):1802-11. doi: 10.1007/s11999-015-4140-1. Epub 2015 Jan 17. Clin Orthop Relat Res. 2015. PMID: 25595096 Free PMC article.
Cited by
-
Dynamic distribution of systemically administered antibiotics in orthopeadically relevant target tissues and settings.APMIS. 2024 Dec;132(12):992-1025. doi: 10.1111/apm.13490. Epub 2024 Nov 12. APMIS. 2024. PMID: 39530161 Free PMC article.
-
Plasma and tissue concentrations of 2 g prophylactic cefazolin prior to lower extremity surgery.Antimicrob Agents Chemother. 2024 Jul 9;68(7):e0049424. doi: 10.1128/aac.00494-24. Epub 2024 May 21. Antimicrob Agents Chemother. 2024. PMID: 38771030 Free PMC article. Clinical Trial.
-
Orthopaedic infections: novel treatment strategies and evolving concepts.OTA Int. 2025 Apr 1;8(2 Suppl):e395. doi: 10.1097/OI9.0000000000000395. eCollection 2025 Apr. OTA Int. 2025. PMID: 40170870 Free PMC article.
References
-
- EUCAST. The European Committee on Antimicrobial Susceptibility Testing. Breakpoint tables for interpretation of MICs and zone diameters, version 13.0. Available at: https://www.eucast.org/clinical_breakpoints. Accessed June 22, 2023.
-
- Hoff WS, Bonadies JA, Cachecho R, Dorlac WC. East Practice Management Guidelines Work Group: update to Practice Management Guidelines for Prophylactic Antibiotic Use in Open Fractures. J Trauma. 2011;70:751-754. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

