Developmental exposure to the Parkinson's disease-associated organochlorine pesticide dieldrin alters dopamine neurotransmission in α-synuclein pre-formed fibril (PFF)-injected mice
- PMID: 37607008
- PMCID: PMC10613968
- DOI: 10.1093/toxsci/kfad086
Developmental exposure to the Parkinson's disease-associated organochlorine pesticide dieldrin alters dopamine neurotransmission in α-synuclein pre-formed fibril (PFF)-injected mice
Abstract
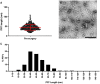
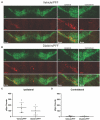
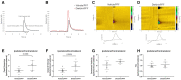
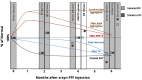
Parkinson's disease (PD) is the fastest-growing neurological disease worldwide, with increases outpacing aging and occurring most rapidly in recently industrialized areas, suggesting a role of environmental factors. Epidemiological, post-mortem, and mechanistic studies suggest that persistent organic pollutants, including the organochlorine pesticide dieldrin, increase PD risk. In mice, developmental dieldrin exposure causes male-specific exacerbation of neuronal susceptibility to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) and synucleinopathy. Specifically, in the α-synuclein (α-syn) pre-formed fibril (PFF) model, exposure leads to increased deficits in striatal dopamine (DA) turnover and motor deficits on the challenging beam. Here, we hypothesized that alterations in DA handling contribute to the observed changes and assessed vesicular monoamine transporter 2 (VMAT2) function and DA release in this dieldrin/PFF 2-hit model. Female C57BL/6 mice were exposed to 0.3 mg/kg dieldrin or vehicle every 3 days by feeding, starting at 8 weeks of age and continuing throughout breeding, gestation, and lactation. Male offspring from independent litters underwent unilateral, intrastriatal injections of α-syn PFFs at 12 weeks of age, and vesicular 3H-DA uptake assays and fast-scan cyclic voltammetry were performed 4 months post-PFF injection. Dieldrin-induced an increase in DA release in striatal slices in PFF-injected animals, but no change in VMAT2 activity. These results suggest that developmental dieldrin exposure increases a compensatory response to synucleinopathy-triggered striatal DA loss. These findings are consistent with silent neurotoxicity, where developmental exposure to dieldrin primes the nigrostriatal striatal system to have an exacerbated response to synucleinopathy in the absence of observable changes in typical markers of nigrostriatal dysfunction and degeneration.
Keywords: Parkinson disease; alpha-synuclein; developmental neurotoxicity; dieldrin; dopamine; pesticides.
© The Author(s) 2023. Published by Oxford University Press on behalf of the Society of Toxicology.
Figures



Similar articles
-
Developmental exposure to the organochlorine pesticide dieldrin causes male-specific exacerbation of α-synuclein-preformed fibril-induced toxicity and motor deficits.Neurobiol Dis. 2020 Jul;141:104947. doi: 10.1016/j.nbd.2020.104947. Epub 2020 May 15. Neurobiol Dis. 2020. PMID: 32422283 Free PMC article.
-
Deficits in basal and evoked striatal dopamine release following alpha-synuclein preformed fibril injection: An in vivo microdialysis study.Eur J Neurosci. 2024 Apr;59(7):1585-1603. doi: 10.1111/ejn.16275. Epub 2024 Feb 14. Eur J Neurosci. 2024. PMID: 38356120
-
Developmental exposure to the pesticide dieldrin alters the dopamine system and increases neurotoxicity in an animal model of Parkinson's disease.FASEB J. 2006 Aug;20(10):1695-7. doi: 10.1096/fj.06-5864fje. Epub 2006 Jun 29. FASEB J. 2006. PMID: 16809432
-
A Combined α-Synuclein/Fibril (SynFib) Model of Parkinson-Like Synucleinopathy Targeting the Nigrostriatal Dopamine System.J Parkinsons Dis. 2022;12(8):2307-2320. doi: 10.3233/JPD-223452. J Parkinsons Dis. 2022. PMID: 36189605 Free PMC article. Review.
-
alpha-Synuclein- and MPTP-generated rodent models of Parkinson's disease and the study of extracellular striatal dopamine dynamics: a microdialysis approach.CNS Neurol Disord Drug Targets. 2010 Aug;9(4):482-90. doi: 10.2174/187152710791556177. CNS Neurol Disord Drug Targets. 2010. PMID: 20522009 Review.
Cited by
-
Developmental origins of Parkinson's disease risk: perinatal exposure to the organochlorine pesticide dieldrin leads to sex-specific DNA modifications in critical neurodevelopmental pathways in the mouse midbrain.bioRxiv [Preprint]. 2024 Apr 29:2024.04.26.590998. doi: 10.1101/2024.04.26.590998. bioRxiv. 2024. Update in: Toxicol Sci. 2024 Oct 1;201(2):263-281. doi: 10.1093/toxsci/kfae091. PMID: 38746441 Free PMC article. Updated. Preprint.
-
Modulating Stress Proteins in Response to Therapeutic Interventions for Parkinson's Disease.Int J Mol Sci. 2023 Nov 12;24(22):16233. doi: 10.3390/ijms242216233. Int J Mol Sci. 2023. PMID: 38003423 Free PMC article. Review.
-
Antagonism of kappa opioid receptors accelerates the development of L-DOPA-induced dyskinesia in a preclinical model of moderate dopamine depletion.Brain Res. 2023 Dec 15;1821:148613. doi: 10.1016/j.brainres.2023.148613. Epub 2023 Sep 30. Brain Res. 2023. PMID: 37783263 Free PMC article.
-
Developmental origins of Parkinson's disease risk: perinatal exposure to the organochlorine pesticide dieldrin leads to sex-specific DNA modifications in critical neurodevelopmental pathways in the mouse midbrain.Toxicol Sci. 2024 Oct 1;201(2):263-281. doi: 10.1093/toxsci/kfae091. Toxicol Sci. 2024. PMID: 38995845 Free PMC article.
-
Pesticide Exposure and Its Association with Parkinson's Disease: A Case-Control Analysis.Cell Mol Neurobiol. 2024 Nov 1;44(1):73. doi: 10.1007/s10571-024-01501-5. Cell Mol Neurobiol. 2024. PMID: 39485576 Free PMC article.
References
-
- Abeliovich A., Schmitz Y., Fariñas I., Choi-Lundberg D., Ho W. H., Castillo P. E., Shinsky N., Verdugo J. M., Armanini M., Ryan A., et al. (2000). Mice lacking a-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron 25, 239–252. - PubMed
-
- Agency for Toxic Substances and Disease Registry (ATSDR) (2022). Toxicological Profile for Aldrin and Dieldrin. U.S. Department of Health and Human Services, Public Health Service, Atlanta, GA. - PubMed
-
- Alves G., Norwegian ParkWest study group, Müller B., Herlofson K., HogenEsch I., Telstad W., Aarsland D., Tysnes O. B., Larsen J. P. (2009). Incidence of parkinson’s disease in Norway: The Norwegian ParkWest study. J. Neurol. Neurosurg. Psychiatry. 80, 851–857. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

