The Effect of CytoSorb on Inflammatory Markers in Critically Ill Patients: A Systematic Review and Meta-Analysis of Randomized Controlled Trials
- PMID: 37607074
- PMCID: PMC10645103
- DOI: 10.1097/CCM.0000000000006007
The Effect of CytoSorb on Inflammatory Markers in Critically Ill Patients: A Systematic Review and Meta-Analysis of Randomized Controlled Trials
Abstract
Objectives: The effectiveness of CytoSorb at removing inflammatory mediators in critically ill patients is controversial.
Data sources: Electronic databases were searched from inception to May 2023.
Study selection: Randomized controlled trials reporting the effects of CytoSorb therapy on inflammatory parameters in critically ill patients with hyperinflammatory conditions were included.
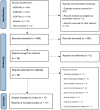
Data extraction: Two authors screened articles for eligibility, extracted data, and assessed the risk of bias, conflicts of interest, and certainty of evidence (CoE). The primary outcome was interleukin (IL)-6 at 1 day after initiation of the therapy. Secondary outcomes included various inflammatory markers at 1, 2, 3, and 5 days and mortality. Data were pooled if at least three trials reported the outcome of interest. We conducted meta-analyses of the data using a random-effects model.
Data synthesis: Seventeen trials ( n = 855) were included. Fourteen trials were judged to have notable concern about conflicts of interest. Seven trials were performed in medical ICU patients with hyperinflammatory conditions and 10 in complex cardiovascular surgery under cardiopulmonary bypass. Hemoadsorption with CytoSorb was not associated with lower IL-6 at 1 day (mean difference -5.98 [95% CI, -30.44 to 18.48] pg/mL), 2 days, 3 days, or 5 days after initiation of the treatment, as well as the concentration of procalcitionin. The levels of C-reactive protein were not lower with CytoSorb at 1, 2, and 3 days. The use of CytoSorb was associated with higher mortality at latest follow-up (relative risk = 1.22 [95% CI, 1.02-1.45]) and at 30 days. CoE ranged from low to very low.
Conclusions: The use of CytoSorb hemoadsorption in a mixed population of critically ill patients with hyperinflammatory conditions does not exhibit a consistent decrease in IL-6 and other inflammatory parameters within the first 5 days of treatment. The significant uncertainty surrounding these findings highlights the need for further investigations.
Trial registration: ClinicalTrials.gov NCT02566525.
Copyright © 2023 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the Society of Critical Care Medicine and Wolters Kluwer Health, Inc.
Conflict of interest statement
The authors have disclosed that they do not have any potential conflicts of interest. Two authors screened articles for eligibility, extracted data, and assessed the risk of bias, conflicts of interest, and certainty of evidence (CoE). The primary outcome was interleukin (IL)-6 at 1 day after initiation of the therapy. Secondary outcomes included various inflammatory markers at 1, 2, 3, and 5 days and mortality. Data were pooled if at least three trials reported the outcome of interest. We conducted meta-analyses of the data using a random-effects model. Seventeen trials ( n = 855) were included. Fourteen trials were judged to have notable concern about conflicts of interest. Seven trials were performed in medical ICU patients with hyperinflammatory conditions and 10 in complex cardiovascular surgery under cardiopulmonary bypass. Hemoadsorption with CytoSorb was not associated with lower IL-6 at 1 day (mean difference −5.98 [95% CI, −30.44 to 18.48] pg/mL), 2 days, 3 days, or 5 days after initiation of the treatment, as well as the concentration of procalcitionin. The levels of C-reactive protein were not lower with CytoSorb at 1, 2, and 3 days. The use of CytoSorb was associated with higher mortality at latest follow-up (relative risk = 1.22 [95% CI, 1.02–1.45]) and at 30 days. CoE ranged from low to very low.
Figures




Comment in
-
Does CytoSorb Pose Unique Challenges to Pooled Estimates in Meta-Analysis?Crit Care Med. 2023 Dec 1;51(12):1819-1821. doi: 10.1097/CCM.0000000000006043. Epub 2023 Nov 16. Crit Care Med. 2023. PMID: 37971336 No abstract available.
-
Effect of CytoSorb on Interleukin-6.Crit Care Med. 2024 Mar 1;52(3):e152-e153. doi: 10.1097/CCM.0000000000006120. Epub 2024 Feb 21. Crit Care Med. 2024. PMID: 38381019 No abstract available.
References
-
- Bonavia A, Groff A, Karamchandani K, et al. : Clinical utility of extracorporeal cytokine hemoadsorption therapy: A literature review. Blood Purif. 2018; 46:337–349 - PubMed
-
- Putzu A, Schorer R, Lopez-Delgado JC, et al. : Blood purification and mortality in sepsis and septic shock: A systematic review and meta-analysis of randomized trials. Anesthesiology. 2019; 131:580–593 - PubMed
-
- CytoSorbents Europe GmbH: The Adsorber. Available at: https://cytosorb-therapy.com/en/the-adsorber/. Accessed February 26, 2023
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials

