PI3K block restores age-dependent neurovascular coupling defects associated with cerebral small vessel disease
- PMID: 37607233
- PMCID: PMC10467353
- DOI: 10.1073/pnas.2306479120
PI3K block restores age-dependent neurovascular coupling defects associated with cerebral small vessel disease
Abstract
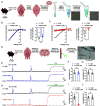
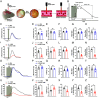
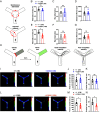
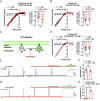
Neurovascular coupling (NVC), a vital physiological process that rapidly and precisely directs localized blood flow to the most active regions of the brain, is accomplished in part by the vast network of cerebral capillaries acting as a sensory web capable of detecting increases in neuronal activity and orchestrating the dilation of upstream parenchymal arterioles. Here, we report a Col4a1 mutant mouse model of cerebral small vessel disease (cSVD) with age-dependent defects in capillary-to-arteriole dilation, functional hyperemia in the brain, and memory. The fundamental defect in aged mutant animals was the depletion of the minor membrane phospholipid phosphatidylinositol 4,5 bisphosphate (PIP2) in brain capillary endothelial cells, leading to the loss of inwardly rectifying K+ (Kir2.1) channel activity. Blocking phosphatidylinositol-3-kinase (PI3K), an enzyme that diminishes the bioavailability of PIP2 by converting it to phosphatidylinositol (3, 4, 5)-trisphosphate (PIP3), restored Kir2.1 channel activity, capillary-to-arteriole dilation, and functional hyperemia. In longitudinal studies, chronic PI3K inhibition also improved the memory function of aged Col4a1 mutant mice. Our data suggest that PI3K inhibition is a viable therapeutic strategy for treating defective NVC and cognitive impairment associated with cSVD.
Keywords: COL4A1; Kir2.1 channels; cerebral small vessel disease; extracellular matrix; functional hyperemia.
Conflict of interest statement
S.E. and D.B.G. have filed a provisional patent for the use of PI3 kinase inhibitors to treat cSVDs.
Figures
Update of
-
PI3K block restores age-dependent neurovascular coupling defects associated with cerebral small vessel disease.bioRxiv [Preprint]. 2023 Mar 6:2023.03.03.531032. doi: 10.1101/2023.03.03.531032. bioRxiv. 2023. Update in: Proc Natl Acad Sci U S A. 2023 Aug 29;120(35):e2306479120. doi: 10.1073/pnas.2306479120. PMID: 36945616 Free PMC article. Updated. Preprint.
References
-
- Dichgans M., Leys D., Vascular cognitive impairment. Circ. Res. 120, 573–591 (2017). - PubMed

