Potent Antifungal Activity of Penta- O-galloyl-β-d-Glucose against Drug-Resistant Candida albicans, Candida auris, and Other Non- albicans Candida Species
- PMID: 37607350
- PMCID: PMC10496123
- DOI: 10.1021/acsinfecdis.3c00113
Potent Antifungal Activity of Penta- O-galloyl-β-d-Glucose against Drug-Resistant Candida albicans, Candida auris, and Other Non- albicans Candida Species
Abstract
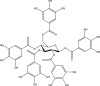
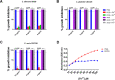
Among fungal pathogens, infections by drug-resistant Candida species continue to pose a major challenge to healthcare. This study aimed to evaluate the activity of the bioactive natural product, penta-O-galloyl-β-d-glucose (PGG) against multidrug-resistant (MDR) Candida albicans, MDR Candida auris, and other MDR non-albicans Candida species. Here, we show that PGG has a minimum inhibitory concentration (MIC) of 0.25-8 μg mL-1 (0.265-8.5 μM) against three clinical strains of C. auris and a MIC of 0.25-4 μg mL-1 (0.265-4.25 μM) against a panel of other MDR Candida species. Our cytotoxicity studies found that PGG was well tolerated by human kidney, liver, and epithelial cells with an IC50 > 256 μg mL-1 (>272 μM). We also show that PGG is a high-capacity iron chelator and that deletion of key iron homeostasis genes in C. albicans rendered strains hypersensitive to PGG. In conclusion, PGG displayed potent anti-Candida activity with minimal cytotoxicity for human cells. We also found that the antifungal activity of PGG is mediated through an iron-chelating mechanism, suggesting that the compound could prove useful as a topical treatment for superficial Candida infections.
Keywords: candidiasis; iron chelation; mechanism of action; natural product.
Conflict of interest statement
The authors declare the following competing financial interest(s): C.L.Q. and L.M. are inventors on a patent pertaining to the use of PGG for the mitigation of fungal infections. L.E.C. and L.W. are a co-founders and shareholders in Bright Angel Therapeutics, a platform company for development of antifungal therapeutics. L.E.C. is a Science Advisor for Kapoose Creek, a company that harnesses the therapeutic potential of fungi.
Figures



Similar articles
-
A chemical screen identifies structurally diverse metal chelators with activity against the fungal pathogen Candida albicans.Microbiol Spectr. 2024 Apr 2;12(4):e0409523. doi: 10.1128/spectrum.04095-23. Epub 2024 Feb 20. Microbiol Spectr. 2024. PMID: 38376363 Free PMC article.
-
Novel Intravenous Immunoglobulin Therapy for the Prevention and Treatment of Candida auris and Candida albicans Disseminated Candidiasis.mSphere. 2023 Feb 21;8(1):e0058422. doi: 10.1128/msphere.00584-22. Epub 2023 Jan 23. mSphere. 2023. PMID: 36688668 Free PMC article.
-
In vitro antifungal activity of Cinnamomum zeylanicum bark and leaf essential oils against Candida albicans and Candida auris.Appl Microbiol Biotechnol. 2020 Oct;104(20):8911-8924. doi: 10.1007/s00253-020-10829-z. Epub 2020 Sep 3. Appl Microbiol Biotechnol. 2020. PMID: 32880694 Free PMC article.
-
New and Promising Chemotherapeutics for Emerging Infections Involving Drug-resistant Non-albicans Candida Species.Curr Top Med Chem. 2019;19(28):2527-2553. doi: 10.2174/1568026619666191025152412. Curr Top Med Chem. 2019. PMID: 31654512 Review.
-
Harnessing Metal Homeostasis Offers Novel and Promising Targets Against Candida albicans.Curr Drug Discov Technol. 2020;17(4):415-429. doi: 10.2174/1570163816666190227231437. Curr Drug Discov Technol. 2020. PMID: 30827249 Review.
Cited by
-
Current Perspectives of Antifungal Therapy: A Special Focus on Candida auris.J Fungi (Basel). 2024 Jun 6;10(6):408. doi: 10.3390/jof10060408. J Fungi (Basel). 2024. PMID: 38921394 Free PMC article. Review.
-
Synthesis and Evaluation of a Chitosan-Based Cationic Hydrogel with Strong Antifungal and Antibiofilm Activities Against Clinical Isolates of Candida auris.Pharmaceuticals (Basel). 2025 Mar 31;18(4):506. doi: 10.3390/ph18040506. Pharmaceuticals (Basel). 2025. PMID: 40283941 Free PMC article.
-
Inhibitory potential of pentagalloyl glucose against efflux pumps in Staphylococcus aureus.RSC Adv. 2025 Aug 20;15(36):29377-29388. doi: 10.1039/d5ra03958d. eCollection 2025 Aug 18. RSC Adv. 2025. PMID: 40860075 Free PMC article.
-
Genomics insights of candidiasis: mechanisms of pathogenicity and drug resistance.Front Microbiol. 2025 Feb 27;16:1531543. doi: 10.3389/fmicb.2025.1531543. eCollection 2025. Front Microbiol. 2025. PMID: 40083780 Free PMC article. Review.
-
In vitro and in vivo activity of 1,2,3,4,6-O-pentagalloyl-glucose against Candida albicans.Antimicrob Agents Chemother. 2025 Mar 5;69(3):e0177524. doi: 10.1128/aac.01775-24. Epub 2025 Jan 24. Antimicrob Agents Chemother. 2025. PMID: 39853121 Free PMC article.
References
-
- Bilal H.; Hou B.; Shafiq M.; Chen X.; Shahid M. A.; Zeng Y. Antifungal susceptibility pattern of Candida isolated from cutaneous candidiasis patients in eastern Guangdong region: A retrospective study of the past 10 years. Front. Microbiol. 2022, 13, 98118110.3389/fmicb.2022.981181. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

