Teplizumab: A Disease-Modifying Therapy for Type 1 Diabetes That Preserves β-Cell Function
- PMID: 37607392
- PMCID: PMC10545553
- DOI: 10.2337/dc23-0675
Teplizumab: A Disease-Modifying Therapy for Type 1 Diabetes That Preserves β-Cell Function
Abstract
Objective: In November 2022, teplizumab-mzwv became the first drug approved to delay the onset of stage 3 type 1 diabetes in adults and children age ≥8 years with stage 2 type 1 diabetes on the basis of data from the pivotal study TN-10.
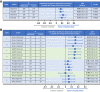
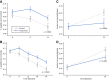
Research design and methods: To provide confirmatory evidence of the effects of teplizumab on preserving endogenous insulin production, an integrated analysis of C-peptide data from 609 patients (n = 375 patients receiving teplizumab and n = 234 control patients) from five clinical trials in stage 3 type 1 diabetes was conducted.
Results: The primary outcome of the integrated analysis, change from baseline in stimulated C-peptide, was significantly improved at years 1 (average increase 0.08 nmol/L; P < 0.0001) and 2 (average increase 0.12 nmol/L; P < 0.0001) after one or two courses of teplizumab. An analysis of exogenous insulin use was also conducted, showing overall reductions of 0.08 (P = 0.0001) and 0.10 units/kg/day (P < 0.0001) at years 1 and 2, respectively. An integrated safety analysis of five clinical trials that enrolled 1,018 patients with stage 2 or 3 type 1 diabetes (∼1,500 patient-years of follow-up for teplizumab-treated patients) was conducted.
Conclusions: These data confirm consistency in the preservation of β-cell function, as measured by C-peptide, across multiple clinical trials. This analysis showed that the most common adverse events included lymphopenia, rash, and headache, a majority of which occurred during and after the first few weeks of teplizumab administration and generally resolved without intervention, consistent with a safety profile characterized by self-limited adverse events after one or two courses of teplizumab treatment.
© 2023 by the American Diabetes Association.
Conflict of interest statement
Figures


References
-
- Xu D, Alegre ML, Varga SS, et al. . In vitro characterization of five humanized OKT3 effector function variant antibodies. Cell Immunol 2000;200:16–26 - PubMed
-
- Kuhn C, Weiner HL. Therapeutic anti-CD3 monoclonal antibodies: from bench to bedside. Immunotherapy 2016;8:889–906 - PubMed
-
- Orthoclone OKT3 [prescribing information] . Raritan, NJ, Centocor Ortho Biotech Products, L.P., 2011. https://www.drugs.com/pro/orthoclone-okt3.html
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

