MicroRNA-9-1 Attenuates Influenza A Virus Replication via Targeting Tankyrase 1
- PMID: 37607510
- PMCID: PMC10601686
- DOI: 10.1159/000532063
MicroRNA-9-1 Attenuates Influenza A Virus Replication via Targeting Tankyrase 1
Abstract
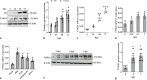
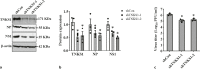
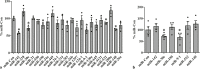
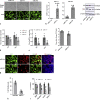
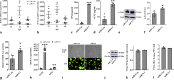
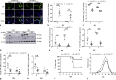
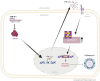
An unstable influenza genome leads to the virus resistance to antiviral drugs that target viral proteins. Thus, identification of host factors essential for virus replication may pave the way to develop novel antiviral therapies. In this study, we investigated the roles of the poly(ADP-ribose) polymerase enzyme, tankyrase 1 (TNKS1), and the endogenous small noncoding RNA, miR-9-1, in influenza A virus (IAV) infection. Increased expression of TNKS1 was observed in IAV-infected human lung epithelial cells and mouse lungs. TNKS1 knockdown by RNA interference repressed influenza viral replication. A screen using TNKS1 3'-untranslation region (3'-UTR) reporter assays and predicted microRNAs identified that miR-9-1 targeted TNKS1. Overexpression of miR-9-1 reduced influenza viral replication in lung epithelial cells as measured by viral mRNA and protein levels as well as virus production. miR-9-1 induced type I interferon production and enhanced the phosphorylation of STAT1 in cell culture. The ectopic expression of miR-9-1 in the lungs of mice by using an adenoviral viral vector enhanced type I interferon response, inhibited viral replication, and reduced susceptibility to IAV infection. Our results indicate that miR-9-1 is an anti-influenza microRNA that targets TNKS1 and enhances cellular antiviral state.
Keywords: Influenza virus; MicroRNA; Poly(ADP-ribose) polymerase; Type I interferon.
© 2023 The Author(s). Published by S. Karger AG, Basel.
Conflict of interest statement
The authors have no conflicts of interest.
Figures



Similar articles
-
MicroRNA-206 inhibits influenza A virus replication by targeting tankyrase 2.Cell Microbiol. 2021 Feb;23(2):e13281. doi: 10.1111/cmi.13281. Epub 2020 Nov 4. Cell Microbiol. 2021. PMID: 33099847 Free PMC article.
-
Influenza A Virus Dysregulates Host Histone Deacetylase 1 That Inhibits Viral Infection in Lung Epithelial Cells.J Virol. 2016 Apr 14;90(9):4614-4625. doi: 10.1128/JVI.00126-16. Print 2016 May. J Virol. 2016. PMID: 26912629 Free PMC article.
-
Lnc-ISG20 Inhibits Influenza A Virus Replication by Enhancing ISG20 Expression.J Virol. 2018 Jul 31;92(16):e00539-18. doi: 10.1128/JVI.00539-18. Print 2018 Aug 15. J Virol. 2018. PMID: 29899085 Free PMC article.
-
Prion Protein is a Novel Modulator of Influenza: Potential Implications for Anti-Influenza Therapeutics.Curr Issues Mol Biol. 2020;37:21-32. doi: 10.21775/cimb.037.021. Epub 2019 Dec 9. Curr Issues Mol Biol. 2020. PMID: 31814573 Review.
-
Host Non-Coding RNA Regulates Influenza A Virus Replication.Viruses. 2021 Dec 29;14(1):51. doi: 10.3390/v14010051. Viruses. 2021. PMID: 35062254 Free PMC article. Review.
Cited by
-
Positive Regulation of Cellular Proteins by Influenza Virus for Productive Infection.Int J Mol Sci. 2025 Apr 10;26(8):3584. doi: 10.3390/ijms26083584. Int J Mol Sci. 2025. PMID: 40332127 Free PMC article. Review.
-
Insight into noncanonical small noncoding RNAs in Influenza A virus infection.Virus Res. 2024 Dec;350:199474. doi: 10.1016/j.virusres.2024.199474. Epub 2024 Sep 27. Virus Res. 2024. PMID: 39326700 Free PMC article.
References
-
- Center of Disease Control . Disease burden of influenza; 2020. Available from: https://www.cdc.gov/flu/about/burden/index.html.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

