Key Prognostic Factors Create a Composite Risk Score to Stratify Patients into High- and Low-Treatment Benefit Groups: A Multicenter, Retrospective Data Analysis of 84 Metastatic Colorectal Cancer Patients Treated with Regorafenib as Part of the CORRECT and CONSIGN Trials
- PMID: 37607525
- PMCID: PMC10614442
- DOI: 10.1159/000531268
Key Prognostic Factors Create a Composite Risk Score to Stratify Patients into High- and Low-Treatment Benefit Groups: A Multicenter, Retrospective Data Analysis of 84 Metastatic Colorectal Cancer Patients Treated with Regorafenib as Part of the CORRECT and CONSIGN Trials
Abstract
Introduction: In further-line mCRC treatment, median progression-free survival (PFS) is rather short, and many patients do not benefit from any antitumor treatment and should therefore be treated according to best-supportive care. A risk score based on standard laboratory values using markers of tumor inflammation aims to define a patient cohort with high treatment benefit and might offer insights into tumor biology. As regorafenib has been dropped off the German market due to an unfavorable risk-benefit ratio, patient selection is key for any further-line treatment option.
Methods: We used Cox regression analysis to determine laboratory markers that are independent prognostic factors of OS and PFS outcome. The influence of these variables was weighted using an estimator, which was calculated using Cox regression analysis. The estimators were implemented as multiplication factors, resulting in a risk score. A cut-off value for the resulting risk values was then determined via Cox regression analysis resulting in a low- and high-risk subgroup.
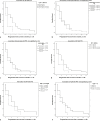
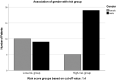
Results: Using data of 82 patients, a risk score identifying long-term survival in patients with last-line mCRC treatment could be calculated. The following parameters were associated with significantly longer survival in multivariate analysis: NLR ≤5 (p = <0.001), AP ≤200 U/L (p = 0.001), CRP ≤3.2 mg/dL (p = <0.001). The following estimator values were used to calculate a risk score: NLR: 0.132 (p = 0.046), AP: 0.004 (p = 0.014), and CRP: 0.032 (p = 0.039). Implementing the estimators as multiplication factors yielded the following risk score: 0.132*NLR + 0.004*AP + 0.032*CRP = Risk value. Cox regression resulted in low- and high-risk subgroups with risk values below and above 1.4, respectively. In the group with a low-risk score (<1.4), patients had a median OS of 10.5 months after initiating regorafenib. Patients with a high-risk score (>1.4) survived only 3.3 months after starting therapy with regorafenib (n = 43, p < 0.001, HR = 3.76).
Conclusions: The presented composite risk score stratifies patients into two prognostic subgroups characterized by standard laboratory values. Patients with signs of systemic inflammation characterized by elevated NLR, AP, and CRP have a high composite risk score and a significant shorter overall survival. Although this score needs to be prospectively validated in larger cohorts, it may be used to stratify patients suitable for further-line treatment studies.
Keywords: Alkaline phosphatase; C-reactive protein; CONSIGN; CORRECT; Metastatic colorectal cancer; Neutrophil leukocyte ratio; Regorafenib; Risk score.
© 2023 The Author(s). Published by S. Karger AG, Basel.
Conflict of interest statement
Sebastian Stintzing received honoraria for talks and/or for advisory function from: Amgen, Bayer, Merck KGaA, Lilly, Sanofi, Roche, Takeda, Taiho, SIRTEX, and Samsung. Volker Heinemann has received honoraria for participating in symposia and advisory boards for Merck, Amgen, Roche, Sanofi, Boehringer Ingelheim, Celgene, Bayer, Pfizer, Sirtex Medical, SERVIER, MSD, Bristol-Myers Squibb, MSD Oncology, Novartis, and Pierre Fabre. He has also received research funding from Merk, Amgen, Roche, Sanofi, Pfizer, Boehringer Ingelheim, Sirtex Medical, and Bayer and travel support from Merck, Roche, Sirtex Medical, Amgen, SERVIER, Shire, MSD, and Bristol-Myers Squibb. None of these activities were related to the content of this article. Jobst C von Einem: honoraria: Merck, Roche, Amgen, Sanofi, Pierre Fabre, Servier, Taiho, BMS, Eisai, and Novartis; consulting or advisory role: Amgen, Pierre-Fabre, BMS, and Servier; travel, accommodations, and expenses: AstraZeneca and Apceth. All remaining authors declared no conflicts of interest.
Figures

References
-
- Grothey A, Van Cutsem E, Sobrero A, Siena S, Falcone A, Ychou M, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet. 2013;381(9863):303–12. 10.1016/S0140-6736(12)61900-X. - DOI - PubMed
-
- Van Cutsem E, Martinelli E, Cascinu S, Sobrero A, Banzi M, Seitz JF, et al. Regorafenib for patients with metastatic colorectal cancer who progressed after standard therapy: results of the large, single-arm, open-label phase IIIb CONSIGN study. Oncologist. 2019;24(2):185–92. 10.1634/theoncologist.2018-0072. - DOI - PMC - PubMed
-
- Hillienhof, Arne. Regorafenib: Onkologen kritisieren marktrücknahme. Deutsches Ärzteblatt. 2016;113(Heft 17):A-797.
-
- Nutzenbewertungsverfahren GB. Zum Wirkstoff Regorafenib nach §35a SGB V; 2016.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

