Timing of Colposcopy and Risk of Cervical Cancer
- PMID: 37607530
- PMCID: PMC10637756
- DOI: 10.1097/AOG.0000000000005313
Timing of Colposcopy and Risk of Cervical Cancer
Abstract
Objective: To quantify the association between time to colposcopy and risk of subsequent cervical cancer.
Methods: A longitudinal analysis of patients aged 21-79 years with an abnormal cervical cancer test result from health care systems in Texas, Massachusetts, and Washington was performed. The outcome was a cervical cancer diagnosis 12 months or more after the abnormal result. The primary analysis compared receipt of colposcopy within 3 months (91 days or less) with receipt of colposcopy at 3-12 months (92-365 days) and no colposcopy within 12 months of the abnormal test result; post hoc analyses compared colposcopy within 12 months (365 days or less) with no colposcopy within 12 months. Associations were assessed with multivariable Cox proportional hazards regression controlling for age, risk status, result severity, and health care system.
Results: Of 17,541 patients, 53.3% of patients received colposcopy within 3 months, 22.2% received colposcopy in 3-12 months, and 24.6% had no colposcopy within 12 months. One hundred forty-seven patients were diagnosed with cervical cancer within 12 months and removed from subsequent analyses. Sixty-five patients (0.4%) were diagnosed with cervical cancer more than 1 year (366 days or more) after the abnormal Pap or human papillomavirus test result. The risk of cervical cancer detection more than 1 year after the abnormal test result was not different in patients who received colposcopy within 3-12 months (hazard ratio [HR] 1.07, 95% CI 0.54-2.12) and higher among patients with no colposcopy within 12 months (HR 2.34, 95% CI 1.33-4.14) compared with patients who had colposcopy within 3 months. Post hoc analyses showed that the risk of cervical cancer diagnosis was 2.29-fold higher among those without colposcopy within 12 months compared with those who received colposcopy within 12 months (95% CI 1.37-3.83); among patients with high-grade cytology results, the risk of cervical cancer detection among those without colposcopy within 12 months was 3.12-fold higher compared with those who received colposcopy within 12 months (95% CI 1.47-6.70).
Conclusion: There was no difference in cervical cancer risk at more than 1 year between patients who received colposcopy within 3 months compared with those who received colposcopy within 3-12 months of an abnormal result. Patients who did not receive colposcopy within 12 months of an abnormal result had a higher risk of subsequent cervical cancer compared with those who received a colposcopy within 12 months.
Copyright © 2023 by the American College of Obstetricians and Gynecologists. Published by Wolters Kluwer Health, Inc. All rights reserved.
Conflict of interest statement
Financial Disclosure Stephanie Alimena reports receiving payment from Roche Diagnostics. Sarah Feldman reports money was paid to her institution from the Society for Improving Diagnosis in Medicine. She received payment from UpToDate. The other authors did not report any potential conflicts of interest.
Figures
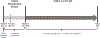
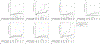
References
-
- Saslow D, Solomon D, Lawson HW, et al. American Cancer Society, American Society for Colposcopy and Cervical Pathology, and American Society for Clinical Pathology screening guidelines for the prevention and early detection of cervical cancer. Am J Clin Pathol. 2012;137(4):516–542. doi: 10.1309/AJCPTGD94EVRSJCG. - DOI - PubMed

