Brain-wide representations of behavior spanning multiple timescales and states in C. elegans
- PMID: 37607537
- PMCID: PMC10836760
- DOI: 10.1016/j.cell.2023.07.035
Brain-wide representations of behavior spanning multiple timescales and states in C. elegans
Abstract
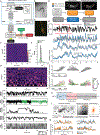
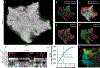
Changes in an animal's behavior and internal state are accompanied by widespread changes in activity across its brain. However, how neurons across the brain encode behavior and how this is impacted by state is poorly understood. We recorded brain-wide activity and the diverse motor programs of freely moving C. elegans and built probabilistic models that explain how each neuron encodes quantitative behavioral features. By determining the identities of the recorded neurons, we created an atlas of how the defined neuron classes in the C. elegans connectome encode behavior. Many neuron classes have conjunctive representations of multiple behaviors. Moreover, although many neurons encode current motor actions, others integrate recent actions. Changes in behavioral state are accompanied by widespread changes in how neurons encode behavior, and we identify these flexible nodes in the connectome. Our results provide a global map of how the cell types across an animal's brain encode its behavior.
Keywords: C. elegans; behavior; brain-wide activity; cell atlas; internal states; neural circuits.
Copyright © 2023 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures





References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

