Skin-type-dependent development of murine mechanosensory neurons
- PMID: 37607547
- PMCID: PMC10615785
- DOI: 10.1016/j.devcel.2023.07.020
Skin-type-dependent development of murine mechanosensory neurons
Abstract
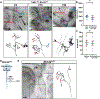
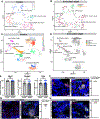
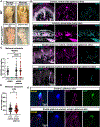
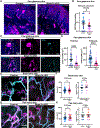
Mechanosensory neurons innervating the skin underlie our sense of touch. Fast-conducting, rapidly adapting mechanoreceptors innervating glabrous (non-hairy) skin form Meissner corpuscles, while in hairy skin, they associate with hair follicles, forming longitudinal lanceolate endings. How mechanoreceptors develop axonal endings appropriate for their skin targets is unknown. We report that mechanoreceptor morphologies across different skin regions are indistinguishable during early development but diverge post-natally, in parallel with skin maturation. Neurons terminating along the glabrous and hairy skin border exhibit hybrid morphologies, forming both Meissner corpuscles and lanceolate endings. Additionally, molecular profiles of neonatal glabrous and hairy skin-innervating neurons largely overlap. In mouse mutants with ectopic glabrous skin, mechanosensory neurons form end-organs appropriate for the altered skin type. Finally, BMP5 and BMP7 are enriched in glabrous skin, and signaling through type I bone morphogenetic protein (BMP) receptors in neurons is critical for Meissner corpuscle morphology. Thus, mechanoreceptor morphogenesis is flexibly instructed by target tissues.
Keywords: axonal development; mechanosensory neurons; morphogenesis; touch end-organs.
Copyright © 2023 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures


Comment in
-
The good, the bald, and the hairy: A mechanosensor meets its fate at the target.Dev Cell. 2023 Oct 23;58(20):2013-2014. doi: 10.1016/j.devcel.2023.08.027. Dev Cell. 2023. PMID: 37875070
Similar articles
-
Specialized mechanoreceptor systems in rodent glabrous skin.J Physiol. 2018 Oct;596(20):4995-5016. doi: 10.1113/JP276608. Epub 2018 Sep 15. J Physiol. 2018. PMID: 30132906 Free PMC article.
-
The good, the bald, and the hairy: A mechanosensor meets its fate at the target.Dev Cell. 2023 Oct 23;58(20):2013-2014. doi: 10.1016/j.devcel.2023.08.027. Dev Cell. 2023. PMID: 37875070
-
The Anatomical Distribution of Mechanoreceptors in Mouse Hind Paw Skin and the Influence of Integrin α1β1 on Meissner-Like Corpuscle Density in the Footpads.Front Neuroanat. 2021 Mar 2;15:628711. doi: 10.3389/fnana.2021.628711. eCollection 2021. Front Neuroanat. 2021. PMID: 33737870 Free PMC article.
-
Mechanosensory perception: are there contributions from bone-associated receptors?Clin Exp Pharmacol Physiol. 2005 Jan-Feb;32(1-2):100-8. doi: 10.1111/j.1440-1681.2005.04136.x. Clin Exp Pharmacol Physiol. 2005. PMID: 15730443 Review.
-
The structure and function of cutaneous sensory receptors.Arch Histol Cytol. 1988 Mar;51(1):1-34. doi: 10.1679/aohc.51.1. Arch Histol Cytol. 1988. PMID: 3137944 Review.
Cited by
-
A Hierarchical Mechanotransduction System: From Macro to Micro.Adv Sci (Weinh). 2024 Mar;11(11):e2302327. doi: 10.1002/advs.202302327. Epub 2023 Dec 25. Adv Sci (Weinh). 2024. PMID: 38145330 Free PMC article. Review.
-
Activity-dependent development of the body's touch receptors.bioRxiv [Preprint]. 2023 Sep 25:2023.09.23.559109. doi: 10.1101/2023.09.23.559109. bioRxiv. 2023. Update in: Neuron. 2025 Jun 4;113(11):1758-1773.e9. doi: 10.1016/j.neuron.2025.04.015. PMID: 37790437 Free PMC article. Updated. Preprint.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

