RNF4 and USP7 cooperate in ubiquitin-regulated steps of DNA replication
- PMID: 37607592
- PMCID: PMC10444366
- DOI: 10.1098/rsob.230068
RNF4 and USP7 cooperate in ubiquitin-regulated steps of DNA replication
Abstract
DNA replication requires precise regulation achieved through post-translational modifications, including ubiquitination and SUMOylation. These modifications are linked by the SUMO-targeted E3 ubiquitin ligases (STUbLs). Ring finger protein 4 (RNF4), one of only two mammalian STUbLs, participates in double-strand break repair and resolving DNA-protein cross-links. However, its role in DNA replication has been poorly understood. Using CRISPR/Cas9 genetic screens, we discovered an unexpected dependency of RNF4 mutants on ubiquitin specific peptidase 7 (USP7) for survival in TP53-null retinal pigment epithelial cells. TP53-/-/RNF4-/-/USP7-/- triple knockout (TKO) cells displayed defects in DNA replication that cause genomic instability. These defects were exacerbated by the proteasome inhibitor bortezomib, which limited the nuclear ubiquitin pool. A shortage of free ubiquitin suppressed the ataxia telangiectasia and Rad3-related (ATR)-mediated checkpoint response, leading to increased cell death. In conclusion, RNF4 and USP7 work cooperatively to sustain a functional level of nuclear ubiquitin to maintain the integrity of the genome.
Keywords: RNF4; STUbL; SUMO; USP7; genome stability; ubiquitin.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

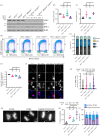
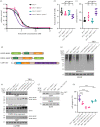



References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

