Role of fatty liver in the epidemic of advanced chronic liver disease among people with HIV: protocol for the Canadian LIVEHIV multicentre prospective cohort
- PMID: 37607785
- PMCID: PMC10445396
- DOI: 10.1136/bmjopen-2023-076547
Role of fatty liver in the epidemic of advanced chronic liver disease among people with HIV: protocol for the Canadian LIVEHIV multicentre prospective cohort
Abstract
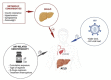
Introduction: Advanced chronic liver disease (ACLD) is a major cause of death for people with HIV (PWH). While viral hepatitis coinfections are largely responsible for this trend, metabolic dysfunction-associated steatotic liver disease (MASLD) is an emerging concern for PWH. We aimed to assess the contribution of MASLD to incident ACLD in PWH.
Methods and analysis: This multicentre prospective observational cohort study will enrol 968 consecutive HIV monoinfected patients from four Canadian sites, excluding subjects with alcohol abuse, liver disease other than MASLD, or ACLD at baseline. Participants will be followed annually for 4 years by clinical evaluation, questionnaires, laboratory testing and Fibroscan to measure liver stiffness measurement (LSM) and controlled attenuation parameter (CAP). The primary outcome will be incidence of ACLD, defined as LSM>10 kPa, by MASLD status, defined as CAP≥285 dB/m with at least one metabolic abnormality, and to develop a score to classify PWH according to their risk of ACLD. Secondary outcomes will include health-related quality of life (HRQoL) and healthcare resource usage. Kaplan-Meier survival method and Cox proportional hazards regression will calculate the incidence and predictors of ACLD, respectively. Propensity score methods and marginal structural models will account for time-varying exposures. We will split the cohort into a training set (to develop the risk score) and a validation set (for validation of the score). HRQoL scores and healthcare resource usage will be compared by MASLD status using generalised linear mixed effects model.
Ethics and dissemination: This protocol has been approved by the ethics committees of all participating institutions. Written informed consent will be obtained from all study participants. The results of this study will be shared through scientific publications and public presentations to advocate for the inclusion of PWH in clinical trials of MASLD-targeted therapies and case-finding of ACLD in PWH.
Keywords: HIV & AIDS; Hepatology; Patient-Centered Care; Quality of Life.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: BL has acted as a speaker and advisory board member for ViiV, Gilead and Merck, and received research funding from ViiV, Merck and Gilead. CTC has received research funding from Merck, Gilead and Tilray, speaker honorarium from Gilead and consultant fees from ViiV Healthcare and Moderna. She has received funding to attend conferences from Gilead and ViiV Healthcare, and cannabinoids from Tilray for use in a clinical trial. SLW has served on advisory board, and spoken at CME event for Merck, Gilead, Viiv, and has received research funds from ViiV, Gilead and Merck. JC has received funding for investigator-initiated research from ViiV Healthcare and remuneration for advisory work from Gilead Canada and ViiV Healthcare. MCMM has served on advisory boards and spoken at CME events for Merck, Gilead and Viiv, and has received research funds from Viiv. GS has acted as speaker for Merck, Gilead, Abbvie, NovoNordisk, Pfizer, served as an advisory board member for Pfizer, Merck, NovoNordisk, Gilead and Intercept and has received unrestricted research funding from Theratecnologies. FC, SS, DK, LRB, WE, EEMM, CP, KM, NP, NK, AdP, J-PR, MBK have no conflict of interests.
Figures
Similar articles
-
MASLD in persons with HIV is associated with high cardiometabolic risk as evidenced by altered advanced lipoprotein profiles and targeted metabolomics.Lipids Health Dis. 2024 Oct 17;23(1):339. doi: 10.1186/s12944-024-02317-4. Lipids Health Dis. 2024. PMID: 39420356 Free PMC article.
-
The effect of weight gain and metabolic dysfunction-associated steatotic liver disease on liver fibrosis progression and regression in people with HIV.AIDS. 2024 Jul 15;38(9):1323-1332. doi: 10.1097/QAD.0000000000003903. Epub 2024 Apr 18. AIDS. 2024. PMID: 38597416 Free PMC article.
-
Distinct metabolic perturbations link liver steatosis and incident CVD in lean but not obese PWH.BMC Med. 2025 Feb 11;23(1):78. doi: 10.1186/s12916-025-03914-5. BMC Med. 2025. PMID: 39934780 Free PMC article.
-
Fatty Liver Disease: Enter the Metabolic Era.Curr HIV/AIDS Rep. 2023 Dec;20(6):405-418. doi: 10.1007/s11904-023-00669-7. Epub 2023 Oct 26. Curr HIV/AIDS Rep. 2023. PMID: 37882965 Review.
-
Dysgeusia in MASLD-related advanced chronic liver disease (ACLD): a silent driver towards the "Bermuda" triangle of malnutrition-sarcopenia-frailty severely affecting prognosis.Nutr J. 2025 Jan 16;24(1):10. doi: 10.1186/s12937-025-01074-z. Nutr J. 2025. PMID: 39819605 Free PMC article. Review.
References
-
- Canada PHA . Estimates of HIV incidence, prevalence and Canada’s progress on meeting the 90-90-90 HIV targets. 2020. Available: https://www.canada.ca/en/public-health/services/publications/diseases-co... [Accessed 15 May 2023].
-
- Global HIV & AIDS Statistics — fact sheet. Available: https://www.unaids.org/en/resources/fact-sheet [Accessed 15 May 2023].
-
- Rosenthal E, Salmon-Céron D, Lewden C, et al. . Liver-related deaths in HIV-infected patients between 1995 and 2005 in the French GERMIVIC joint study group network (Mortavic 2005 study in collaboration with the Mortalité 2005 survey, ANRS En19). HIV Med 2009;10:282–9. 10.1111/j.1468-1293.2008.00686.x - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
