Effect of tapered versus stable treatment with tumour necrosis factor inhibitors on disease flares in patients with rheumatoid arthritis in remission: a randomised, open label, non-inferiority trial
- PMID: 37607809
- PMCID: PMC10579188
- DOI: 10.1136/ard-2023-224476
Effect of tapered versus stable treatment with tumour necrosis factor inhibitors on disease flares in patients with rheumatoid arthritis in remission: a randomised, open label, non-inferiority trial
Abstract
Objectives: Many patients with rheumatoid arthritis (RA) require treatment with tumour necrosis factor inhibitor (TNFi) to reach remission. It is debated whether tapering of TNFi to discontinuation should be considered in sustained remission. The aim of ARCTIC REWIND TNFi was to assess the effect of tapering TNFi to withdrawal compared with stable treatment on the risk of disease activity flares in patients with RA in remission ≥1 year.
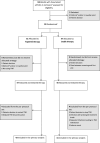
Methods: This randomised, open-label, non-inferiority trial was undertaken at nine Norwegian rheumatology departments. Patients with RA in remission ≥12 months on stable TNFi therapy were allocated by computer-based block-randomisation to tapering to discontinuation of TNFi or stable TNFi. Conventional synthetic disease-modifying antirheumatic co-medication was unchanged. The primary endpoint was disease flare during the 12-month study period (non-inferiority margin 20%), assessed in the per-protocol population.
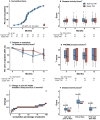
Results: Between June 2013 and January 2019, 99 patients were enrolled and 92 received the allocated treatment strategy. Eighty-four patients were included in the per-protocol population. In the tapering TNFi group, 27/43 (63%) experienced a flare during 12 months, compared with 2/41 (5%) in the stable TNFi group; risk difference (95% CI) 58% (42% to 74%). The tapering strategy was not non-inferior to continued stable treatment. The number of total/serious adverse events was 49/3 in the tapering group, 57/2 in the stable group.
Conclusion: In patients with RA in remission for more than 1 year while using TNFi, an increase in flare rate was reported in those who tapered TNFi to discontinuation. However, most regained remission after reinstatement of full-dose treatment.
Trial registration numbers: EudraCT: 2012-005275-14 and clinicaltrials.gov: NCT01881308.
Keywords: arthritis, rheumatoid; treatment; tumor necrosis factor inhibitors.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: SL, NPS, JS and EM report grants from The Research Council of Norway and from The South-Eastern Norway Regional Health Authority during the conduct of the study. ABA reports personal fees from AbbVie, personal fees from Eli Lilly, personal fees from Novartis, personal fees from Pfizer, outside the submitted work. ICO, HF, CS, ÅL, IMH have nothing to disclose. CAH reports personal fees from Novartis, outside the submitted work. GB reports personal fees from Novartis, personal fees from UCB, outside the submitted work. HH reports personal fees from Novartis, outside the submitted work. TU reports personal fees from Galapagos, personal fees from Lilly, personal fees from Novartis, personal fees from Pfizer, personal fees from UCB, outside the submitted work. DHS reports that Abbvie donated drugs for a trial, that Amgen donated drugs for a trial, grants from Corrona, grants from Janssen, grants from Pfizer, grants from Roche/Genentech, outside the submitted work. DvdH reports personal fees from AbbVie, personal fees from Amgen, personal fees from Astellas, personal fees from AstraZeneca, personal fees from BMS, personal fees from Boehringer Ingelheim, personal fees from Celgene, personal fees from Cyxone, personal fees from Daichii, personal fees from Eisai, personal fees from Eli Lilly, personal fees from Galapagos, personal fees from Gilead, personal fees from GSK, personal fees from Janssen, personal fees from Merck, personal fees from Novartis, personal fees from Pfizer, personal fees from Regeneron, personal fees from Roche, personal fees from Sanofi, personal fees from Takeda, personal fees from UCB Pharma, outside the submitted work; and is Director Imaging Rheumatology BV. TKK reports grants and personal fees from AbbVie, personal fees from Biogen, personal fees from Celltrion, personal fees from Egis, personal fees from Lilly, grants and personal fees from MSD, personal fees from Mylan, personal fees from Hikma, grants and personal fees from Novartis, personal fees from Oktal, personal fees from Orion Pharma, grants and personal fees from Pfizer, personal fees from Roche, personal fees from Sandoz, personal fees from Sanofi, grants and personal fees from UCB, grants from BMS, outside the submitted work. EAH reports grants from The Research Council of Norway, grants from The South-Eastern Norway Regional Health Authority, during the conduct of the study; personal fees from Pfizer, personal fees from AbbVie, personal fees from Janssen-Cilag, personal fees from Gilead, personal fees from UCB Pharma, personal fees from Celgene, personal fees from Lilly, personal fees from Roche, outside the submitted work.
Figures

Comment in
-
In RA in remission for >1 y, tapering TNFi to discontinuation did not meet noninferiority criteria compared with stable TNFi for disease flare at 12 mo.Ann Intern Med. 2023 Dec;176(12):JC139. doi: 10.7326/J23-0095. Epub 2023 Dec 5. Ann Intern Med. 2023. PMID: 38048575
References
-
- Uhrenholt L, Christensen R, Dinesen WKH, et al. Risk of flare after tapering or withdrawal of biologic/targeted synthetic disease-modifying anti-rheumatic drugs in patients with rheumatoid arthritis or axial spondyloarthritis: a systematic review and meta-analysis. Rheumatology (Oxford) 2022;61:3107–22. 10.1093/rheumatology/keab902 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

