Evaluation of the determinants for improved pluripotency induction and maintenance by engineered SOX17
- PMID: 37607832
- PMCID: PMC10516664
- DOI: 10.1093/nar/gkad597
Evaluation of the determinants for improved pluripotency induction and maintenance by engineered SOX17
Abstract
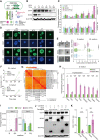
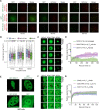
An engineered SOX17 variant with point mutations within its DNA binding domain termed SOX17FNV is a more potent pluripotency inducer than SOX2, yet the underlying mechanism remains unclear. Although wild-type SOX17 was incapable of inducing pluripotency, SOX17FNV outperformed SOX2 in mouse and human pluripotency reprogramming. In embryonic stem cells, SOX17FNV could replace SOX2 to maintain pluripotency despite considerable sequence differences and upregulated genes expressed in cleavage-stage embryos. Mechanistically, SOX17FNV co-bound OCT4 more cooperatively than SOX2 in the context of the canonical SoxOct DNA element. SOX2, SOX17, and SOX17FNV were all able to bind nucleosome core particles in vitro, which is a prerequisite for pioneer transcription factors. Experiments using purified proteins and in cellular contexts showed that SOX17 variants phase-separated more efficiently than SOX2, suggesting an enhanced ability to self-organise. Systematic deletion analyses showed that the N-terminus of SOX17FNV was dispensable for its reprogramming activity. However, the C-terminus encodes essential domains indicating multivalent interactions that drive transactivation and reprogramming. We defined a minimal SOX17FNV (miniSOX) that can support reprogramming with high activity, reducing the payload of reprogramming cassettes. This study uncovers the mechanisms behind SOX17FNV-induced pluripotency and establishes engineered SOX factors as powerful cell engineering tools.
© The Author(s) 2023. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures





Similar articles
-
Cancer-associated missense mutations enhance the pluripotency reprogramming activity of OCT4 and SOX17.FEBS J. 2020 Jan;287(1):122-144. doi: 10.1111/febs.15076. Epub 2019 Oct 17. FEBS J. 2020. PMID: 31569299
-
Conversion of Sox17 into a pluripotency reprogramming factor by reengineering its association with Oct4 on DNA.Stem Cells. 2011 Jun;29(6):940-51. doi: 10.1002/stem.639. Stem Cells. 2011. PMID: 21472822
-
Sox transcription factors require selective interactions with Oct4 and specific transactivation functions to mediate reprogramming.Stem Cells. 2013 Dec;31(12):2632-46. doi: 10.1002/stem.1522. Stem Cells. 2013. PMID: 23963638
-
SOX17 in cellular reprogramming and cancer.Semin Cancer Biol. 2020 Dec;67(Pt 1):65-73. doi: 10.1016/j.semcancer.2019.08.008. Epub 2019 Aug 13. Semin Cancer Biol. 2020. PMID: 31419525 Review.
-
Biological importance of OCT transcription factors in reprogramming and development.Exp Mol Med. 2021 Jun;53(6):1018-1028. doi: 10.1038/s12276-021-00637-4. Epub 2021 Jun 11. Exp Mol Med. 2021. PMID: 34117345 Free PMC article. Review.
Cited by
-
Advancements in personalized stem cell models for aging-related neurodegenerative disorders.Neural Regen Res. 2024 Nov 1;19(11):2333-2334. doi: 10.4103/NRR.NRR-D-23-01793. Epub 2024 Jan 31. Neural Regen Res. 2024. PMID: 38526261 Free PMC article. No abstract available.
-
An acidic residue within the OCT4 dimerization interface of SOX17 is necessary and sufficient to overcome its pluripotency-inducing activity.Stem Cell Reports. 2025 Mar 11;20(3):102398. doi: 10.1016/j.stemcr.2025.102398. Epub 2025 Feb 6. Stem Cell Reports. 2025. PMID: 39919754 Free PMC article.
-
Molecular basis for SOX2-dependent regulation of super-enhancer activity.Nucleic Acids Res. 2023 Dec 11;51(22):11999-12019. doi: 10.1093/nar/gkad908. Nucleic Acids Res. 2023. PMID: 37930832 Free PMC article.
-
Enhanced Activities of OCT4 and SOX2 Promote Epigenetic Reprogramming by Shortening G1 Phase.Adv Sci (Weinh). 2025 Aug;12(32):e15528. doi: 10.1002/advs.202415528. Epub 2025 May 31. Adv Sci (Weinh). 2025. PMID: 40448624 Free PMC article.
-
The emergence of Sox and POU transcription factors predates the origins of animal stem cells.Nat Commun. 2024 Nov 14;15(1):9868. doi: 10.1038/s41467-024-54152-x. Nat Commun. 2024. PMID: 39543096 Free PMC article.
References
-
- Takahashi K., Yamanaka S.. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006; 126:663–676. - PubMed
-
- Thier M.C., Hommerding O., Panten J., Pinna R., García-González D., Berger T., Wörsdörfer P., Assenov Y., Scognamiglio R., Przybylla A.et al. .. Identification of embryonic neural plate border stem cells and their generation by direct reprogramming from adult human blood cells. Cell Stem Cell. 2019; 24:166–182. - PubMed
-
- Nakagawa M., Koyanagi M., Tanabe K., Takahashi K., Ichisaka T., Aoi T., Okita K., Mochiduki Y., Takizawa N., Yamanaka S.. Generation of induced pluripotent stem cells without Myc from mouse and human fibroblasts. Nat. Biotechnol. 2008; 26:101–106. - PubMed
-
- An Z., Liu P., Zheng J., Si C., Li T., Chen Y., Ma T., Zhang M.Q., Zhou Q., Ding S.. Sox2 and Klf4 as the functional core in pluripotency induction without exogenous Oct4. Cell Rep. 2019; 29:1986–2000. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

