Association between maternal hyperglycemia in pregnancy and offspring anthropometry in early childhood: the pandora wave 1 study
- PMID: 37608089
- PMCID: PMC10599996
- DOI: 10.1038/s41366-023-01366-6
Association between maternal hyperglycemia in pregnancy and offspring anthropometry in early childhood: the pandora wave 1 study
Abstract
Background: In-utero hyperglycemia exposure influences later cardiometabolic risk, although few studies include women with pre-existing type 2 diabetes (T2D) or assess maternal body mass index (BMI) as a potential confounder.
Objective: To explore the association of maternal T2D and gestational diabetes mellitus (GDM) with childhood anthropometry, and the influence of maternal BMI on these associations.
Methods: The PANDORA cohort comprises women (n = 1138) and children (n = 1163). Women with GDM and T2D were recruited from a hyperglycemia in pregnancy register, and women with normoglycemia from the community. Wave 1 follow-up included 423 children, aged 1.5-5 years (median follow-up age 2.5 years). Multivariable linear regression assessed associations between maternal antenatal variables, including BMI and glycemic status, with offspring anthropometry (weight, height, BMI, skinfold thicknesses, waist, arm and head circumferences).
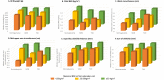
Results: Greater maternal antenatal BMI was associated with increased anthropometric measures in offspring independent of maternal glycemic status. After adjustment, including for maternal BMI, children exposed to maternal GDM had lower mean weight (-0.54 kg, 95% CI: -0.99, -0.11), BMI (-0.55 kg/m2, 95% CI: -0.91, -0.20), head (-0.52 cm, 95% CI: -0.88, -0.16) and mid-upper arm (-0.32 cm, 95% CI: -0.63, -0.01) circumferences, and greater mean suprailiac skinfold (0.78 mm, 95% CI: 0.13, 1.43), compared to children exposed to normoglycemia. Adjustment for maternal BMI strengthened the negative association between GDM and child weight, BMI and circumferences. Children exposed to maternal T2D had smaller mean head circumference (-0.82 cm, 95% CI: -1.33, -0.31) than children exposed to normoglycemia. Maternal T2D was no longer associated with greater child mean skinfolds (p = 0.14) or waist circumference (p = 0.18) after adjustment for maternal BMI.
Conclusions: Children exposed to GDM had greater suprailiac skinfold thickness than unexposed children, despite having lower mean weight, BMI and mid-upper arm circumference, and both GDM and T2D were associated with smaller mean head circumference. Future research should assess whether childhood anthropometric differences influence lifetime cardiometabolic and neurodevelopmental risk.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

