Colloidal InAs Tetrapods: Impact of Surfactants on the Shape Control
- PMID: 37608781
- PMCID: PMC10450814
- DOI: 10.1021/jacs.3c03906
Colloidal InAs Tetrapods: Impact of Surfactants on the Shape Control
Abstract
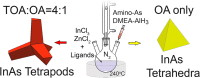
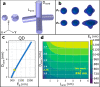
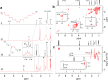
We have approached the synthesis of colloidal InAs nanocrystals (NCs) using amino-As and ligands that are different from the commonly employed oleylamine (OA). We found that carboxylic and phosphonic acids led only to oxides, whereas tri-n-octylphosphine, dioctylamine, or trioctylamine (TOA), when employed as the sole ligands, yielded InAs NCs with irregular sizes and a broad size distribution. Instead, various combinations of TOA and OA delivered InAs NCs with good control over the size distribution, and the TOA:OA volume ratio of 4:1 generated InAs tetrapods with arm length of 5-6 nm. Contrary to tetrapods of II-VI materials, which have a zinc-blende core and wurtzite arms, these NCs are entirely zinc-blende, with arms growing along the ⟨111⟩ directions. They feature a narrow excitonic peak at ∼950 nm in absorption and a weak photoluminescence emission at 1050 nm. Our calculations indicated that the bandgap of the InAs tetrapods is mainly governed by the size of their core and not by their arm lengths when these are longer than ∼3 nm. Nuclear magnetic resonance analyses revealed that InAs tetrapods are mostly passivated by OA with only a minor fraction of TOA. Molecular dynamics simulations showed that OA strongly binds to the (111) facets whereas TOA weakly binds to the edges and corners of the NCs and their combined use (at high TOA:OA volume ratios) promotes growth along the ⟨111⟩ directions, eventually forming tetrapods. Our work highlights the use of mixtures of ligands as a means of improving control over InAs NCs size and size distribution.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- Wijaya H.; Darwan D.; Lim K. R. G.; Wang T.; Khoo K. H.; Tan Z.-K. Large-Stokes-Shifted Infrared-Emitting InAs-In(Zn)P-ZnSe-ZnS Giant-Shell Quantum Dots by One-Pot Continuous-Injection Synthesis. Chem. Mater. 2019, 31, 2019–2026. 10.1021/acs.chemmater.8b05023. - DOI
-
- Enright M. J.; Jasrasaria D.; Hanchard M. M.; Needell D. R.; Phelan M. E.; Weinberg D.; McDowell B. E.; Hsiao H.-W.; Akbari H.; Kottwitz M.; Potter M. M.; Wong J.; Zuo J.-M.; Atwater H. A.; Rabani E.; Nuzzo R. G. Role of Atomic Structure on Exciton Dynamics and Photoluminescence in NIR Emissive InAs/InP/ZnSe Quantum Dots. J. Phys. Chem. C 2022, 126, 7576–7587. 10.1021/acs.jpcc.2c01499. - DOI
-
- Goossens S.; Navickaite G.; Monasterio C.; Gupta S.; Piqueras J. J.; Pérez R.; Burwell G.; Nikitskiy I.; Lasanta T.; Galán T.; Puma E.; Centeno A.; Pesquera A.; Zurutuza A.; Konstantatos G.; Koppens F. Broadband Image Sensor Array Based on Graphene-Cmos Integration. Nat. Photonics 2017, 11, 366–371. 10.1038/nphoton.2017.75. - DOI
-
- Geiregat P.; Houtepen A. J.; Sagar L. K.; Infante I.; Zapata F.; Grigel V.; Allan G.; Delerue C.; Van Thourhout D.; Hens Z. Continuous-Wave Infrared Optical Gain and Amplified Spontaneous Emission at Ultralow Threshold by Colloidal HgTe Quantum Dots. Nat. Mater. 2018, 17, 35–42. 10.1038/nmat5000. - DOI - PubMed

