Oral Tetrahydrocannabinol (THC):Cannabinoid (CBD) Cannabis Extract Adjuvant for Reducing Chemotherapy-Induced Nausea and Vomiting (CINV): A Randomized, Double-Blinded, Placebo-Controlled, Crossover Trial
- PMID: 37608911
- PMCID: PMC10440684
- DOI: 10.2147/IJWH.S401938
Oral Tetrahydrocannabinol (THC):Cannabinoid (CBD) Cannabis Extract Adjuvant for Reducing Chemotherapy-Induced Nausea and Vomiting (CINV): A Randomized, Double-Blinded, Placebo-Controlled, Crossover Trial
Abstract
Objective: To evaluate the effects of tetrahydrocannabinol (THC):cannabinoid (CBD) (1:1) oil in reducing chemotherapy-induced nausea and vomiting (CINV) in gynecologic cancer patients who received moderate-to-high emetogenic chemotherapy.
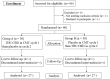
Material and method: This was a randomized, double-blinded, crossover and placebo-controlled trial. The study was conducted at the Gynecologic Oncology Units, Bhumibol Adulyadej Hospital (BAH), Royal Thai Air Force, Bangkok, Thailand, between August and November 2022. Participants had gynecologic cancer and received moderate-to-high emetogenic chemotherapy. Subjects were randomized and divided into two groups (A and B) based on the block of four randomization method. In the first cycle, groups A and B received THC:CBD extract oil 1:1 (TCEO) and placebo before chemotherapy administration. In the second cycle, groups A and B received placebo and TCEO before chemotherapy administration. Both groups received per protocol antiemetic medication during chemotherapy. Nausea score and side effects were recorded.
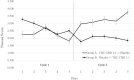
Results: A total of 60 cases were recruited. After exclusion, 54 cases were included in the study. The mean age of participants was 54.4 years. The mean body mass index (BMI) was 26.5 kg/m2. Fifty-nine (21/54) percent cases were the advanced stages of cancer. The nausea score of TCEO and placebo groups were 2.11 and 2.99, respectively (P < 0.05). More than half of the participants (36/54) reported dizziness and sedation side effects. Dry mouth, confusion, anxiety, and palpitation of both groups were comparable.
Conclusion: The cannabinoid extract (THC:CBD) was an appropriate adjuvant agent to reduce CINV in patients with gynecologic cancer who received high-emetogenic chemotherapy. Dizziness and sedation were the major side effects.
Keywords: CBD; CINV; THC; cannabis.
© 2023 Sukpiriyagul et al.
Conflict of interest statement
There is no conflict of interest in this work.
Figures
References
-
- Friedlander ML, Markman M. Chemotherapy. In: Berek JS, Hecker NF, editors. Berek & Hacker’s Gynecologic Oncology. 16th ed. Philadelphia: Wolters Kluwer; 2020:58–84.
-
- National Comprehensive Cancer Network. Antiemesis (Version 2.2022). Available from: https://www.nccn.org/professionals/physician_gls/pdf/antiemesis.pdf. Accessed October 05, 2022.
LinkOut - more resources
Full Text Sources