Safety, Reactogenicity, Immunogenicity, and Dose Selection of 10-Valent Extraintestinal Pathogenic Escherichia coli Bioconjugate Vaccine (VAC52416) in Adults Aged 60-85 Years in a Randomized, Multicenter, Interventional, First-in-Human, Phase 1/2a Study
- PMID: 37608916
- PMCID: PMC10442062
- DOI: 10.1093/ofid/ofad417
Safety, Reactogenicity, Immunogenicity, and Dose Selection of 10-Valent Extraintestinal Pathogenic Escherichia coli Bioconjugate Vaccine (VAC52416) in Adults Aged 60-85 Years in a Randomized, Multicenter, Interventional, First-in-Human, Phase 1/2a Study
Abstract
Background: ExPEC10V is a bioconjugate vaccine containing O-antigen polysaccharides of 10 extraintestinal pathogenic Escherichia coli (ExPEC) serotypes. This phase 1/2a study (NCT03819049) assessed the safety, reactogenicity, and immunogenicity of ExPEC10V (VAC52416) to prevent invasive E coli disease in elderly adults.
Methods: The observer-blind, active-controlled design included a 28-day screening, vaccination, 181-day follow-up, and 1-year follow-up. Participants (60-85 years of age) were randomized to ExPEC10V low dose (antigen dose range, 4-8 µg), ExPEC10V medium dose (4-16 µg), or ExPEC10V high dose (8-16 µg); 4-valent ExPEC vaccine (ExPEC4V); or 13-valent pneumococcal conjugate vaccine (PCV13). The incidence of adverse events (AEs; solicited, day 15; unsolicited, day 30; serious AEs, day 181) and immunogenicity (electrochemiluminescent-based assay [ECL] and multiplex opsonophagocytic assay [MOPA]) were assessed. Optimal ExPEC10V dose was determined from safety data through day 30 and an immunogenicity dose selection algorithm based on day 15 ECL and MOPA results.
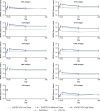
Results: A total of 416 participants were included (median age, 64.0 years; 54.8% female). The incidences of solicited local and systemic AEs were, respectively, 44.2% and 39.4% for low-dose, 52.9% and 46.1% for medium-dose, 57.7% and 45.2% for high-dose ExPEC10V, and 74.1% and 48.1% for PCV13. Five serious AEs, not vaccine related, were reported. The ECL revealed a robust antibody response to ExPEC10V through year 1. Opsonophagocytic killing activity was detected against all but serotype O8; this lack of response against serotype O8 was linked to low assay sensitivity. Based on the totality of data, high-dose ExPEC10V was considered optimal.
Conclusions: ExPEC10V was well tolerated and immunogenic in elderly adults against all but serotype O8.
Keywords: E coli; bacteremia; extraintestinal pathogenic E coli; invasive E coli disease; vaccine.
© The Author(s) 2023. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest. C. A. F. is an employee of Johnson County Clin-Trials, which has performed contracted research for Janssen Research & Development, LLC. M. S., J. D., B. S., O. G., T. A. D., G. v. d. V., J. P., and W. H. are employees of Janssen. D. A. was an employee of Janssen at the time of the analysis.
Figures


Similar articles
-
Safety, reactogenicity, and immunogenicity of different doses of 10-valent Extraintestinal Pathogenic Escherichia Coli (ExPEC10V) bioconjugate vaccine (VAC52416) in healthy Japanese adults aged 60-85 years in a randomized, double-blind, phase 1 study.J Infect Chemother. 2025 Jan;31(1):102514. doi: 10.1016/j.jiac.2024.09.003. Epub 2024 Sep 5. J Infect Chemother. 2025. PMID: 39243886 Clinical Trial.
-
A randomized phase 1/2a trial of ExPEC10V vaccine in adults with a history of UTI.NPJ Vaccines. 2024 Jun 14;9(1):106. doi: 10.1038/s41541-024-00885-1. NPJ Vaccines. 2024. PMID: 38877036 Free PMC article.
-
Safety, tolerability and immunogenicity of the ExPEC4V (JNJ-63871860) vaccine for prevention of invasive extraintestinal pathogenic Escherichia coli disease: A phase 1, randomized, double-blind, placebo-controlled study in healthy Japanese participants.Hum Vaccin Immunother. 2018;14(9):2150-2157. doi: 10.1080/21645515.2018.1474316. Epub 2018 Jun 28. Hum Vaccin Immunother. 2018. PMID: 29771596 Free PMC article. Clinical Trial.
-
Safety and immunogenicity of a vaccine for extra-intestinal pathogenic Escherichia coli (ESTELLA): a phase 2 randomised controlled trial.Lancet Infect Dis. 2019 Jun;19(6):631-640. doi: 10.1016/S1473-3099(18)30803-X. Epub 2019 May 9. Lancet Infect Dis. 2019. PMID: 31079947 Clinical Trial.
-
Immunogenicity and safety of the 13-valent pneumococcal conjugate vaccine compared to 23-valent pneumococcal polysaccharide in immunocompetent adults: A systematic review and meta-analysis.Vaccine. 2019 Feb 14;37(8):1021-1029. doi: 10.1016/j.vaccine.2019.01.014. Epub 2019 Jan 23. Vaccine. 2019. PMID: 30685252
Cited by
-
Urinary tract infections: pathogenesis, host susceptibility and emerging therapeutics.Nat Rev Microbiol. 2025 Feb;23(2):72-86. doi: 10.1038/s41579-024-01092-4. Epub 2024 Sep 9. Nat Rev Microbiol. 2025. PMID: 39251839 Review.
-
The Promising Potential of Reverse Vaccinology-Based Next-Generation Vaccine Development over Conventional Vaccines against Antibiotic-Resistant Bacteria.Vaccines (Basel). 2023 Jul 20;11(7):1264. doi: 10.3390/vaccines11071264. Vaccines (Basel). 2023. PMID: 37515079 Free PMC article. Review.
-
Antimicrobial resistance and vaccines in Enterobacteriaceae including extraintestinal pathogenic Escherichia coli and Klebsiella pneumoniae.NPJ Antimicrob Resist. 2025 Apr 28;3(1):34. doi: 10.1038/s44259-025-00100-8. NPJ Antimicrob Resist. 2025. PMID: 40295787 Free PMC article. Review.
-
Vaccines and monoclonal antibodies to prevent healthcare-associated bacterial infections.Clin Microbiol Rev. 2024 Sep 12;37(3):e0016022. doi: 10.1128/cmr.00160-22. Epub 2024 Aug 9. Clin Microbiol Rev. 2024. PMID: 39120140 Free PMC article. Review.
-
Recent advances in the biosynthesis of polysaccharide-based antimicrobial glycoconjugate vaccines.Front Microbiol. 2025 Jan 29;15:1457908. doi: 10.3389/fmicb.2024.1457908. eCollection 2024. Front Microbiol. 2025. PMID: 39943962 Free PMC article. Review.
References
-
- Dale AP, Woodford N. Extra-intestinal pathogenic Escherichia coli (ExPEC): disease, carriage and clones. J Infect 2015; 71:615–26. - PubMed
-
- Russo TA, Johnson JR. Medical and economic impact of extraintestinal infections due to Escherichia coli: focus on an increasingly important endemic problem. Microbes Infect 2003; 5:449–56. - PubMed
-
- Vila J, Sáez-López E, Johnson JR, et al. . Escherichia coli: an old friend with new tidings. FEMS Microbiol Rev 2016; 40:437–63. - PubMed

