Non-targeted metabolomics analysis reveals the mechanism of arbuscular mycorrhizal symbiosis regulating the cold-resistance of Elymus nutans
- PMID: 37608949
- PMCID: PMC10440431
- DOI: 10.3389/fmicb.2023.1134585
Non-targeted metabolomics analysis reveals the mechanism of arbuscular mycorrhizal symbiosis regulating the cold-resistance of Elymus nutans
Abstract
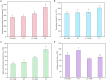
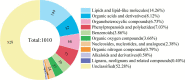
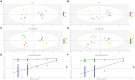
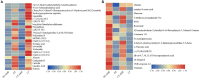
Elymus nutans is a perennial grass of the Gramineae family. Due to its cold-resistance and nutrition deficiency tolerance, it has been applied to the ecological restoration of degraded alpine grassland on the Qinghai-Tibet Plateau. As an important symbiotic microorganism, arbuscular mycorrhizal fungi (AMF) have been proven to have great potential in promoting the growth and stress resistance of Gramineae grasses. However, the response mechanism of the AMF needs to be clarified. Therefore, in this study, Rhizophagus irregularis was used to explore the mechanism regulating cold resistance of E. nutans. Based on pot experiments and metabolomics, the effects of R. irregularis were investigated on the activities of antioxidant enzyme and metabolites in the roots of E. nutans under cold stress (15/10°C, 16/8 h, day/night). The results showed that lipids and lipid molecules are the highest proportion of metabolites, accounting for 14.26% of the total metabolites. The inoculation with R. irregularis had no significant effects on the activities of antioxidant enzyme in the roots of E. nutans at room temperature. However, it can significantly change the levels of some lipids and other metabolites in the roots. Under cold stress, the antioxidant enzyme activities and the levels of some metabolites in the roots of E. nutans were significantly changed. Meanwhile, most of these metabolites were enriched in the pathways related to plant metabolism. According to the correlation analysis, the activities of antioxidant enzyme were closely related to the levels of some metabolites, such as flavonoids and lipids. In conclusion, AMF may regulate the cold-resistance of Gramineae grasses by affecting plant metabolism, antioxidant enzyme activities and antioxidant-related metabolites like flavonoids and lipids. These results can provide some basis for studying the molecular mechanism of AMF regulating stress resistance of Gramineae grasses.
Keywords: Gramineae grasses; arbuscular mycorrhizal fungi; metabolomics; resistance mechanism; stress resistance.
Copyright © 2023 Zhang, Qi, Lu, Zhou, Wang, Li, Zheng, Fan, Zhou, Wang and Wu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The reviewer JU declared a past co-authorship with the author HuZ to the handling editor.
Figures





References
-
- Agati G., Cerovic Z. G., Pinelli P., Tattini M. (2011). Light-induced accumulation of ortho-dihydroxylated flavonoids as non-destructively monitored by chlorophyll fluorescence excitation techniques. Environ. Exp. Bot. 73, 3–9. doi: 10.1016/j.envexpbot.2010.10.002 - DOI
-
- Aroca R., Ruiz-Lozano J. M., Zamarreno A. M., Paz J. A., Garcia-Mina J. M., Pozo M. J., et al. (2013). Arbuscular mycorrhizal symbiosis influences strigolactone production under salinity and alleviates salt stress in lettuce plants. J. Plant Physiol. 170, 47–55. doi: 10.1016/j.jplph.2012.08.020 - DOI - PubMed
-
- Bao G. S., Suetsugu K. J., Wang H. S., Yao X., Liu L., Ou J., et al. (2015). Effects of the hemiparasitic plant Pedicularis kansuensis on plant community structure in a degraded grassland. Ecol. Res. 30, 507–515. doi: 10.1007/s11284-015-1248-4 - DOI
LinkOut - more resources
Full Text Sources
Research Materials

