Dodecafluoropentane Emulsion as a Radiosensitizer in Glioblastoma Multiforme
- PMID: 37609003
- PMCID: PMC10441549
- DOI: 10.1158/2767-9764.CRC-22-0433
Dodecafluoropentane Emulsion as a Radiosensitizer in Glioblastoma Multiforme
Abstract
Purpose: Glioblastoma multiforme (GBM) is a hypoxic tumor resistant to radiotherapy. The purpose of this study was to assess the safety and efficacy of a novel oxygen therapeutic, dodecafluoropentane emulsion (DDFPe), in chemoradiation treatment of GBM.
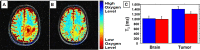
Experimental design: In this multicenter phase Ib/II dose-escalation study, patients were administered DDFPe via intravenous infusion (0.05, 0.10, or 0.17 mL/kg) while breathing supplemental oxygen prior to each 2 Gy fraction of radiotherapy (30 fractions over 6 weeks). Patients also received standard-of-care chemotherapy [temozolomide (TMZ)]. Serial MRI scans were taken to monitor disease response. Adverse events were recorded and graded. TOLD (tissue oxygenation level-dependent) contrast MRI was obtained to validate modulation of tumor hypoxia.
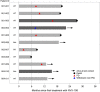
Results: Eleven patients were enrolled. DDFPe combined with radiotherapy and TMZ was well tolerated in most patients. Two patients developed delayed grade 3 radiation necrosis during dose escalation, one each at 0.1 and 0.17 mL/kg of DDFPe. Subsequent patients were treated at the 0.1 mL/kg dose level. Kaplan-Meier analysis showed a median overall survival of 19.4 months and a median progression-free survival of 9.6 months, which compares favorably to historical controls. Among 6 patients evaluable for TOLD MRI, a statistically significant reduction in tumor T1 was observed after DDFPe treatment.
Conclusions: This trial, although small, showed that the use of DDFPe as a radiosensitizer in patients with GBM was generally safe and may provide a survival benefit. This is also the first time than TOLD MRI has shown reversal of tumor hypoxia in a clinical trial in patients. The recommended dose for phase II evaluation is 0.1 mL/kg DDFPe.Trial Registration: NCT02189109.
Significance: This study shows that DDFPe can be safely administered to patients, and it is the first-in-human study to show reversal of hypoxia in GBM as measured by TOLD MRI. This strategy is being used in a larger phase II/III trial which will hopefully show a survival benefit by adding DDFPe during the course of fractionated radiation and concurrent chemotherapy.
© 2023 The Authors; Published by the American Association for Cancer Research.
Figures

References
-
- Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. . Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 2005;352:987–96. - PubMed
-
- Vaupel P, Hockel M, Mayer A. Detection and characterization of tumor hypoxia using pO2 histography. Antioxid Redox Signal 2007;9:1221–35. - PubMed
-
- Rampling R, Cruickshank G, Lewis AD, Fitzsimmons SA, Workman P. Direct measurement of pO2 distribution and bioreductive enzymes in human malignant brain tumors. Int J Radiat Oncol Biol Phys 1994;29:427–31. - PubMed
-
- Collingridge DR, Piepmeier JM, Rockwell S, Knisely JP. Polarographic measurements of oxygen tension in human glioma and surrounding peritumoural brain tissue. Radiother Oncol 1999;53:127–31. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

