This is a preprint.
Unleashing the Power of NR4A1 Degradation as a Novel Strategy for Cancer Immunotherapy
- PMID: 37609171
- PMCID: PMC10441411
- DOI: 10.1101/2023.08.09.552650
Unleashing the Power of NR4A1 Degradation as a Novel Strategy for Cancer Immunotherapy
Update in
-
PROTAC-mediated NR4A1 degradation as a novel strategy for cancer immunotherapy.J Exp Med. 2024 Mar 4;221(3):e20231519. doi: 10.1084/jem.20231519. Epub 2024 Feb 9. J Exp Med. 2024. PMID: 38334978 Free PMC article.
Abstract
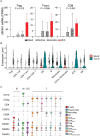
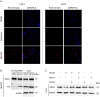
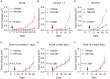
An effective cancer therapy requires both killing cancer cells and targeting tumor-promoting pathways or cell populations within the tumor microenvironment (TME). We purposely search for molecules that are critical for multiple tumor-promoting cell types and identified nuclear receptor subfamily 4 group A member 1 (NR4A1) as one such molecule. NR4A1 has been shown to promote the aggressiveness of cancer cells and maintain the immune suppressive TME. Using genetic and pharmacological approaches, we establish NR4A1 as a valid therapeutic target for cancer therapy. Importantly, we have developed the first-of-its kind proteolysis-targeting chimera (PROTAC, named NR-V04) against NR4A1. NR-V04 effectively degrades NR4A1 within hours of treatment in vitro and sustains for at least 4 days in vivo, exhibiting long-lasting NR4A1-degradation in tumors and an excellent safety profile. NR-V04 leads to robust tumor inhibition and sometimes eradication of established melanoma tumors. At the mechanistic level, we have identified an unexpected novel mechanism via significant induction of tumor-infiltrating (TI) B cells as well as an inhibition of monocytic myeloid derived suppressor cells (m-MDSC), two clinically relevant immune cell populations in human melanomas. Overall, NR-V04-mediated NR4A1 degradation holds promise for enhancing anti-cancer immune responses and offers a new avenue for treating various types of cancer.
Keywords: NR4A1; PROTAC degrader; cancer immunotherapy; immune modulation; melanoma.
Conflict of interest statement
This project was partially supported by an iAward from Sanofi. W.Z., G.Z., D.Z., Y.X., and L.W. hold a patent related to the compound used in this study. X.L., D.S., K.R.G. are Sanofi Employees and holding Sanofi stocks.
Figures






References
-
- Zeng H, Qin L, Zhao D, Tan X, Manseau EJ, Van Hoang M, Senger DR, Brown LF, Nagy JA, Dvorak HF. Orphan nuclear receptor TR3/Nur77 regulates VEGF-A-induced angiogenesis through its transcriptional activity. J Exp Med. 2006;203(3):719–29. Epub 2006/03/08. doi: 10.1084/jem.20051523. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
