This is a preprint.
Impact of KRAS Mutations and Co-mutations on Clinical Outcomes in Pancreatic Ductal Adenocarcinoma
- PMID: 37609177
- PMCID: PMC10441514
- DOI: 10.21203/rs.3.rs-3195257/v1
Impact of KRAS Mutations and Co-mutations on Clinical Outcomes in Pancreatic Ductal Adenocarcinoma
Update in
-
Impact of KRAS mutations and co-mutations on clinical outcomes in pancreatic ductal adenocarcinoma.NPJ Precis Oncol. 2024 Feb 3;8(1):27. doi: 10.1038/s41698-024-00505-0. NPJ Precis Oncol. 2024. PMID: 38310130 Free PMC article.
Abstract
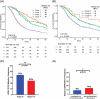
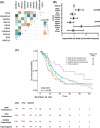
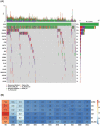
The relevance of KRAS mutation alleles to clinical outcome remains inconclusive in pancreatic adenocarcinoma (PDAC). We conducted a retrospective study of 803 PDAC patients (42% with metastatic disease) at MD Anderson Cancer Center. Overall survival (OS) analysis demonstrated that KRAS mutation status and subtypes were prognostic (p<0.001). Relative to patients with KRAS wildtype tumors (median OS 38 months), patients with KRASG12R had a similar OS (median 34 months), while patients with KRASQ61 and KRASG12D mutated tumors had shorter OS (median 20 months [HR: 1.9, 95% CI 1.2-3.0, p=0.006] and 22 months [HR: 1.7, 95% CI 1.3-2.3, p<0.001], respectively). There was enrichment of KRASG12D mutation in metastatic tumors (34% vs 24%, OR: 1.7, 95% CI 1.2-2.4, p=0.001) and enrichment of KRASG12R in well and moderately differentiated tumors (14% vs 9%, OR: 1.7, 95% CI 1.05-2.99, p=0.04). Similar findings were observed in the external validation cohort (PanCAN's Know Your Tumor® dataset, n=408).
Keywords: Alleles; Clinical outcome; Co-mutations; KRAS; Mutation; OS; PDAC; Pancreatic cancer; Subtypes; Survival.
Conflict of interest statement
Additional Declarations: There is a conflict of interest Dan Zhao is an advisory board member for Affini-T and she has clinical trial contract with CARsgen and Mirati. Disclosures and Competing interests: This data has not been previously presented. COI disclosures pending from authors.
Figures




References
-
- Mizrahi JD, Surana R, Valle JW, Shroff RT. Pancreatic cancer. Lancet. 2020;395(10242):2008–2020. - PubMed
-
- Conroy T, Desseigne F, Ychou M, et al. FOLFIRINOX versus Gemcitabine for Metastatic Pancreatic Cancer. New England Journal of Medicine. 2011;364(19):1817–1825. - PubMed
-
- Tempero MA, Malafa MP, Al-Hawary M, et al. Pancreatic Adenocarcinoma, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2021;19(4):439–457. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

