This is a preprint.
Utilization of 5'-deoxy-nucleosides as Growth Substrates by Extraintestinal Pathogenic E. coli via the Dihydroxyacetone Phosphate Shunt
- PMID: 37609188
- PMCID: PMC10441430
- DOI: 10.1101/2023.08.10.552779
Utilization of 5'-deoxy-nucleosides as Growth Substrates by Extraintestinal Pathogenic E. coli via the Dihydroxyacetone Phosphate Shunt
Abstract
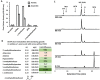
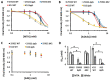
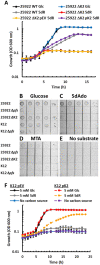
All organisms utilize S-adenosyl-l-methionine (SAM) as a key co-substrate for methylation of biological molecules, synthesis of polyamines, and radical SAM reactions. When these processes occur, 5'-deoxy-nucleosides are formed as byproducts such as S-adenosyl-l-homocysteine (SAH), 5'-methylthioadenosine (MTA), and 5'-deoxyadenosine (5dAdo). One of the most prevalent pathways found in bacteria for the metabolism of MTA and 5dAdo is the DHAP shunt, which converts these compounds into dihydroxyacetone phosphate (DHAP) and 2-methylthioacetaldehyde or acetaldehyde, respectively. Previous work has shown that the DHAP shunt can enable methionine synthesis from MTA or serve as an MTA and 5dAdo detoxification pathway. Here we show that in Extraintestinal Pathogenic E. coil (ExPEC), the DHAP shunt serves none of these roles in any significant capacity, but rather physiologically functions as an assimilation pathway for use of MTA and 5dAdo as growth substrates. This is further supported by the observation that when MTA is the substrate for the ExPEC DHAP shunt, the sulfur components is not significantly recycled back to methionine, but rather accumulates as 2-methylthioethanol, which is slowly oxidized non-enzymatically under aerobic conditions. While the pathway is active both aerobically and anaerobically, it only supports aerobic ExPEC growth, suggesting that it primarily functions in oxygenic extraintestinal environments like blood and urine versus the predominantly anoxic gut. This reveals a heretofore overlooked role of the DHAP shunt in carbon assimilation and energy metabolism from ubiquitous SAM utilization byproducts and suggests a similar role may occur in other pathogenic and non-pathogenic bacteria with the DHAP shunt.
Figures


Similar articles
-
Escherichia coli possessing the dihydroxyacetone phosphate shunt utilize 5'-deoxynucleosides for growth.Microbiol Spectr. 2024 Apr 2;12(4):e0308623. doi: 10.1128/spectrum.03086-23. Epub 2024 Mar 5. Microbiol Spectr. 2024. PMID: 38441472 Free PMC article.
-
Two Distinct Aerobic Methionine Salvage Pathways Generate Volatile Methanethiol in Rhodopseudomonas palustris.mBio. 2018 Apr 10;9(2):e00407-18. doi: 10.1128/mBio.00407-18. mBio. 2018. PMID: 29636438 Free PMC article.
-
A bifunctional salvage pathway for two distinct S-adenosylmethionine by-products that is widespread in bacteria, including pathogenic Escherichia coli.Mol Microbiol. 2020 May;113(5):923-937. doi: 10.1111/mmi.14459. Epub 2020 Feb 20. Mol Microbiol. 2020. PMID: 31950558 Free PMC article.
-
5-Deoxyadenosine Metabolism: More than "Waste Disposal".Microb Physiol. 2021;31(3):248-259. doi: 10.1159/000516105. Epub 2021 Jun 14. Microb Physiol. 2021. PMID: 34126623 Review.
-
Metabolic characteristics and importance of the universal methionine salvage pathway recycling methionine from 5'-methylthioadenosine.IUBMB Life. 2009 Dec;61(12):1132-42. doi: 10.1002/iub.278. IUBMB Life. 2009. PMID: 19946895 Review.
References
-
- Russo T.A. and Johnson J.R., Medical and economic impact of extraintestinal infections due to Escherichia coli: focus on an increasingly important endemic problem. Microbes and infection, 2003. 5(5): p. 449–456. - PubMed
-
- Zhang L. and Foxman B., Molecular epidemiology of Escherichia coli mediated urinary tract infections. Frontiers in Bioscience-Landmark, 2003. 8(5): p. 235–244. - PubMed
-
- Owrangi B., et al., Invasion and translocation of uropathogenic Escherichia coli isolated from urosepsis and patients with community-acquired urinary tract infection. European Journal of Clinical Microbiology & Infectious Diseases, 2018. 37: p. 833–839. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
