This is a preprint.
Within-Individual Organization of the Human Cerebral Cortex: Networks, Global Topography, and Function
- PMID: 37609246
- PMCID: PMC10441314
- DOI: 10.1101/2023.08.08.552437
Within-Individual Organization of the Human Cerebral Cortex: Networks, Global Topography, and Function
Update in
-
Organization of the human cerebral cortex estimated within individuals: networks, global topography, and function.J Neurophysiol. 2024 Jun 1;131(6):1014-1082. doi: 10.1152/jn.00308.2023. Epub 2024 Mar 15. J Neurophysiol. 2024. PMID: 38489238 Free PMC article.
Abstract
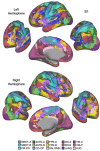
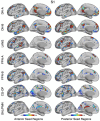
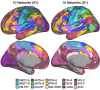
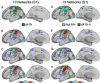
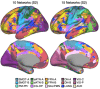
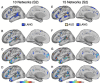
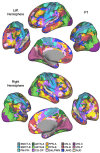
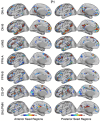
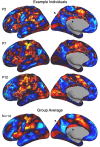
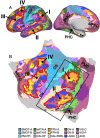
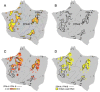
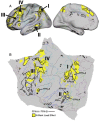
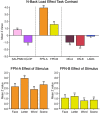
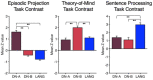
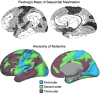
The human cerebral cortex is populated by specialized regions that are organized into networks. Here we estimated networks using a Multi-Session Hierarchical Bayesian Model (MS-HBM) applied to intensively sampled within-individual functional MRI (fMRI) data. The network estimation procedure was initially developed and tested in two participants (each scanned 31 times) and then prospectively applied to 15 new participants (each scanned 8 to 11 times). Detailed analysis of the networks revealed a global organization. Locally organized first-order sensory and motor networks were surrounded by spatially adjacent second-order networks that also linked to distant regions. Third-order networks each possessed regions distributed widely throughout association cortex. Moreover, regions of distinct third-order networks displayed side-by-side juxtapositions with a pattern that repeated similarly across multiple cortical zones. We refer to these as Supra-Areal Association Megaclusters (SAAMs). Within each SAAM, two candidate control regions were typically adjacent to three separate domain-specialized regions. Independent task data were analyzed to explore functional response properties. The somatomotor and visual first-order networks responded to body movements and visual stimulation, respectively. A subset of the second-order networks responded to transients in an oddball detection task, consistent with a role in orienting to salient or novel events. The third-order networks, including distinct regions within each SAAM, showed two levels of functional specialization. Regions linked to candidate control networks responded to working memory load across multiple stimulus domains. The remaining regions within each SAAM did not track working memory load but rather dissociated across language, social, and spatial / episodic processing domains. These results support a model of the cerebral cortex in which progressively higher-order networks nest outwards from primary sensory and motor cortices. Within the apex zones of association cortex there is specialization of large-scale networks that divides domain-flexible from domain-specialized regions repeatedly across parietal, temporal, and prefrontal cortices. We discuss implications of these findings including how repeating organizational motifs may emerge during development.
Figures

























Similar articles
-
Organization of the human cerebral cortex estimated within individuals: networks, global topography, and function.J Neurophysiol. 2024 Jun 1;131(6):1014-1082. doi: 10.1152/jn.00308.2023. Epub 2024 Mar 15. J Neurophysiol. 2024. PMID: 38489238 Free PMC article.
-
Human striatal association megaclusters.J Neurophysiol. 2024 Jun 1;131(6):1083-1100. doi: 10.1152/jn.00387.2023. Epub 2024 Mar 20. J Neurophysiol. 2024. PMID: 38505898 Free PMC article.
-
Situating the left-lateralized language network in the broader organization of multiple specialized large-scale distributed networks.J Neurophysiol. 2020 Nov 1;124(5):1415-1448. doi: 10.1152/jn.00753.2019. Epub 2020 Sep 23. J Neurophysiol. 2020. PMID: 32965153 Free PMC article.
-
From sensation to cognition.Brain. 1998 Jun;121 ( Pt 6):1013-52. doi: 10.1093/brain/121.6.1013. Brain. 1998. PMID: 9648540 Review.
-
Integrated technology for evaluation of brain function and neural plasticity.Phys Med Rehabil Clin N Am. 2004 Feb;15(1):263-306. doi: 10.1016/s1047-9651(03)00124-4. Phys Med Rehabil Clin N Am. 2004. PMID: 15029909 Review.
References
-
- Amlien IK, Fjell AM, Tamnes CK, Grydeland H, Krogsrud SK, Chaplin TA, Rosa MG, Walhovd KB. Organizing principles of human cortical development—thickness and area from 4 to 30 years: Insights from comparative primate neuroanatomy. Cereb Cortex 26:257–267, 2016. - PubMed
-
- Amunts K, Schleicher A, Bürgel U, Mohlberg H, Uylings HB, Zilles K. Broca's region revisited: Cytoarchitecture and intersubject variability. J Comp Neurol 412:319–341, 1999. - PubMed
-
- Amunts K, Malikovic A, Mohlberg H, Schormann T, Zilles K. Brodmann's areas 17 and 18 brought into stereotaxic space—where and how variable? Neuroimage 11:66–84, 2000. - PubMed
-
- Amunts K, Mohlberg H, Bludau S, Zilles K. Julich-Brain: A 3D probabilistic atlas of the human brain’s cytoarchitecture. Science 369:988–992, 2020. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
