This is a preprint.
Illuminating the Function of the Orphan Transporter, SLC22A10 in Humans and Other Primates
- PMID: 37609337
- PMCID: PMC10441401
- DOI: 10.1101/2023.08.08.552553
Illuminating the Function of the Orphan Transporter, SLC22A10 in Humans and Other Primates
Update in
-
Illuminating the function of the orphan transporter, SLC22A10, in humans and other primates.Nat Commun. 2024 May 23;15(1):4380. doi: 10.1038/s41467-024-48569-7. Nat Commun. 2024. PMID: 38782905 Free PMC article.
Abstract
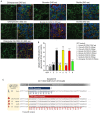
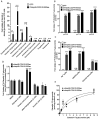
SLC22A10 is classified as an orphan transporter with unknown substrates and function. Here we describe the discovery of the substrate specificity and functional characteristics of SLC22A10. The human SLC22A10 tagged with green fluorescent protein was found to be absent from the plasma membrane, in contrast to the SLC22A10 orthologs found in great apes. Estradiol-17β-glucuronide accumulated in cells expressing great ape SLC22A10 orthologs (over 4-fold, p<0.001). In contrast, human SLC22A10 displayed no uptake function. Sequence alignments revealed two amino acid differences including a proline at position 220 of the human SLC22A10 and a leucine at the same position of great ape orthologs. Site-directed mutagenesis yielding the human SLC22A10-P220L produced a protein with excellent plasma membrane localization and associated uptake function. Neanderthal and Denisovan genomes show human-like sequences at proline 220 position, corroborating that SLC22A10 were rendered nonfunctional during hominin evolution after the divergence from the pan lineage (chimpanzees and bonobos). These findings demonstrate that human SLC22A10 is a unitary pseudogene and was inactivated by a missense mutation that is fixed in humans, whereas orthologs in great apes transport sex steroid conjugates.
Figures



References
-
- Yee SW, Stecula A, Chien HC, Zou L, Feofanova EV, van Borselen M, Cheung KWK, Yousri NA, Suhre K, Kinchen JM, Boerwinkle E, Irannejad R, Yu B, Giacomini KM. Unraveling the functional role of the orphan solute carrier, SLC22A24 in the transport of steroid conjugates through metabolomic and genome-wide association studies. PLoS Genet. 2019;15(9):e1008208. Epub 2019/09/26. doi: 10.1371/journal.pgen.1008208. competing interests exist. - DOI - PMC - PubMed
-
- Girardi E, Agrimi G, Goldmann U, Fiume G, Lindinger S, Sedlyarov V, Srndic I, Gurtl B, Agerer B, Kartnig F, Scarcia P, Di Noia MA, Lineiro E, Rebsamen M, Wiedmer T, Bergthaler A, Palmieri L, Superti-Furga G. Epistasis-driven identification of SLC25A51 as a regulator of human mitochondrial NAD import. Nat Commun. 2020;11(1):6145. Epub 2020/12/03. doi: 10.1038/s41467-020-19871-x. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
