Comparison of SARS-CoV-2 seroconversion in children with chronic diseases with healthy children and adults during the first waves of the COVID-19 pandemic
- PMID: 37609364
- PMCID: PMC10440688
- DOI: 10.3389/fped.2023.1210181
Comparison of SARS-CoV-2 seroconversion in children with chronic diseases with healthy children and adults during the first waves of the COVID-19 pandemic
Abstract
Background: Infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is clinically diverse, and children have a low risk of developing severe coronavirus disease 2019 (COVID-19). However, children with chronic diseases have a potentially increased risk.
Methods: We performed a prospective surveillance study with longitudinal serum SARS-CoV-2 anti-nucleocapsid antibody quantification and questionnaires in pediatric tertiary care patients during the first waves of the COVID-19 pandemic (November 2020-September 2021). The results were compared with those of healthy children and adults from the same geographic area.
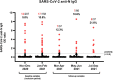
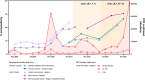
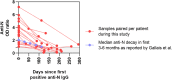
Results: We obtained 525 samples from 362 patients (M/F ratio of 1.3:1; median age of 11.1 years) comprising children with immune-suppressive or immune-modulating drugs (32.9%), inborn errors of immunity (23.5%), type 1 diabetes mellitus (15.2%), and rheumatic diseases (11.9%). A total of 51 (9.7%) samples were seropositive among 37/351 children (10.5%). Seropositivity increased from 5.8% in November-December 2020 to 21.6% in July-September 2021. Compared with adults, a longitudinal analysis revealed reduced seroprevalence but similar kinetics as in children from the same country. Demographic or social variables and disease characteristics did not correlate with seropositivity. Being obese and household contact with COVID-19-infected individuals significantly increased the odds of infection. The majority of seropositive patients had mild symptoms (21/37). One-third were asymptomatic and/or unaware of having COVID-19 (10/37). Four patients (4/37) needed hospitalization, with good clinical outcomes.
Conclusions: Although harboring a chronic disease, we observed a low SARS-CoV-2 incidence in a cohort of pediatric tertiary care patients, comparable with healthy children during the first year of the pandemic. Infection was mostly associated with mild symptoms.
Keywords: COVID-19; SARS-CoV-2; chronic diseases; serology; tertiary care pediatric patients.
© 2023 Hoste, Prytula, Dehoorne, De Bruyne, Van Biervliet, De Waele, Maes, Bordon, Vanlander, Claes, Vande Walle, Schelstraete, Van daele and Haerynck.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

Similar articles
-
Comparison of Seroconversion in Children and Adults With Mild COVID-19.JAMA Netw Open. 2022 Mar 1;5(3):e221313. doi: 10.1001/jamanetworkopen.2022.1313. JAMA Netw Open. 2022. PMID: 35262717 Free PMC article.
-
Seroprevalence of anti-SARS-CoV-2 antibodies among children and their parents in Greece.Eur J Pediatr. 2023 Jan;182(1):439-449. doi: 10.1007/s00431-022-04681-8. Epub 2022 Nov 16. Eur J Pediatr. 2023. PMID: 36383284 Free PMC article.
-
Effectiveness and cost-effectiveness of four different strategies for SARS-CoV-2 surveillance in the general population (CoV-Surv Study): a structured summary of a study protocol for a cluster-randomised, two-factorial controlled trial.Trials. 2021 Jan 8;22(1):39. doi: 10.1186/s13063-020-04982-z. Trials. 2021. PMID: 33419461 Free PMC article.
-
Analysis of the impact of the SARS-CoV-2 infection on the pediatric population hospitalized during the pandemic in the Greater Paris University Hospitals.Front Pediatr. 2023 Feb 27;11:1044352. doi: 10.3389/fped.2023.1044352. eCollection 2023. Front Pediatr. 2023. PMID: 36923274 Free PMC article.
-
Seroepidemiology of SARS-CoV-2 in pediatric population during a 16-month period prior to vaccination.J Med Virol. 2022 May;94(5):2174-2180. doi: 10.1002/jmv.27608. Epub 2022 Jan 31. J Med Virol. 2022. PMID: 35064572 Free PMC article.
Cited by
-
Post-discharge follow-up of pediatric COVID-19 patients: insights into serological dynamics.Front Microbiol. 2024 Jul 9;15:1427327. doi: 10.3389/fmicb.2024.1427327. eCollection 2024. Front Microbiol. 2024. PMID: 39044945 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Miscellaneous

